
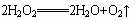
��.��ͼ��һ��ʵ��������װ�ã�ij����С������������װ�ÿ�����ȡ�������Ȼ������壬��ѡ�õ��Լ��У�A.ŨH2SO4��B.Ũ���ᣬC.ʳ�Σ�D.MnO2��?E.H2O2(aq)?��F.KClO3��G.KMnO4��Һ��������������⣺?
(1)��Ҫ�����Ʊ�����������Ӧѡ��___________(ѡ����)��?
(2)��Ҫ�����Ʊ�����HCl��Ӧѡ��___________(ѡ����)��?
��.��֪H2O2(aq)�����ԣ�Ҳ��Ư�����ã�����MnO2�Ĵ��¿��Լ��ٷֽ⣺?
![]()
ijѧ���������Ϻ�ָ��Na2O2��ˮ��Ӧʱ��������NaOH��H2O2��?
![]()
�˷�Ӧ�ų�����������ʹH2O2�����ֽ⣬����O2��?
![]()
�ù��̵��ܷ�Ӧ����ʽ��Ϊ��?
![]()
��ͬѧ���ڵĿ�����ȤС������֤����������ʶ��ʵ�����ṩ������������?ҩƷ��??
�������Թܡ���ͷ�ιܡ��ձ���ҩ�ס�����?
ҩƷ��Na2O2��ˮ����̪��Һ��ʯ����Һ��MnO2?
�������Ǹÿ���С��ij�Ա���������ʵ����̣���д��ʵ�������ʵ����ۡ�
��������.(1)��ȡO2�����������з�Ӧԭ��
![]()
(2)��ȡHCl��������ŨH2SO4����ˮ�Ժ�����ˮ�ų������ȵ��ص㣬��ŨH2SO4����Ũ�����У�ʹŨ�����лӷ���HCl���塣
��.�����öԱ�ʵ�飬һʵ���ڢ۷�Ӧ����ǰ�����̪����ʵ���ڢ۷�Ӧ����������̪(������MnO2��ʹH2O2��ȫ�ֽ�)����һʵ���ȱ�����ɫ����ʵ��ֻ��첻��ɫ���������ͬѧ��ʶ��ȷ��
�𰸣���.(1)DE��(2)AB
��.����ҩ��ȡ�����������ƹ�������Թ��У�������μ���ˮ�����ٲ������壬������ˮϡ�ͣ��۽���Һһ��Ϊ������һ֧�Թ��е�2�η�̪��Һ����Һ�ȱ�죬Լ����Ӻ��ɫ��ȥ������һ֧�Թ����ȼ�����MnO2����������ų�������Ӧ��ɺ��ټӷ�̪1��2�Σ���Һ���ɫ�����־ò���ɫ���ɴ�˵����ͬѧ�Ĺ۵���ȷ����û��������������ȷ��


 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| X | 496 | 4562 | 6912 | 9543 |
| Y | 738 | 1451 | 7733 | 10540 |
| Z | 578 | 1817 | 2745 | 11578 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������b�н���sp
������b�н���sp 3�ӻ���ԭ���У�
3�ӻ���ԭ���У�
 ����1L�ܱ������м���4-��������100g�����������������������������������Ƽ���
����1L�ܱ������м���4-��������100g�����������������������������������Ƽ��� �����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣�
�����״�250mL���ܼ������ܷ⣻�ȳ��뵪����Ȼ�����������һ��ѹ��������������ѹ�����¶ȵ������¼��ⷴӦ����ַ�Ӧ����ȴ�����ˣ�ϴ�ӣ������ռ�64��65����֣�| ��1�¶� | ��2����ѹ�� | ��3�������� | ��4�������� | |||||||||||||||
| ��� | �¶�/�� | ת����/% | ѡ�� ��/% |
��Ӧʱ��/h | ��� | ����ѹ��/MPa | ѡ����/% | ��Ӧʱ��/h | ��� | ����������/g | ѡ����/% | ��Ӧʱ��/h | ��� | ��������/% | ѡ����/% | |||
| �� | 40 | δ��ȫ | 99.6 | 6 | �� | 0.5 | 99.6 | 3.7 | �� | 2 | 98.25 | 5 | �� | 0.0 | 84.3 | |||
| �� | 60 | 100 | 99.7 | 4 | �� | 1.0 | 99.7 | 2 | �� | 4 | 99.20 | 2.2 | �� | 0.3 | 99.3 | |||
| �� | 80 | 100 | 99.6 | 2.45 | �� | 1.5 | 99.2 | 1.6 | �� | 6 | 99.60 | 1.9 | �� | 0.5 | 99.7 | |||
| �� | 100 | 100 | 99.6 | 2 | �� | 2.0 | 96.4 | 1.15 | �� | 8 | 99.60 | 1.4 | �� | 0.7 | 99.6 | |||
| �� | 120 | 100 | 98.6 | 1.7 | �� | �� | 10 | 99.10 | 1.4 | �� | 1.2 | 99.7 | ||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
A.����������һ�ֽྻ����������Դ����������(��Ҫ�ɷ�ΪCO��CO2��H2��)��H2��ϣ����ϳɼ״��������������õķ���֮һ��
(1)������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�ء�д����̬Znԭ�ӵĺ�������Ų�ʽ_________________________��
(2)���ݵȵ���ԭ����д��CO���ӵĽṹʽ______________________��
(3)�״��������ɵõ���ȩ����ȩ������Cu(OH)2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����_____________________����ȩ������̼ԭ�ӹ�����ӻ�����Ϊ_____________________��
�ڼ�ȩ���ӵĿռ乹����_____________________��1 mol��ȩ�����ЦҼ�����ĿΪ_____________________��

����1��Cu2O������(�ṹ��ͼ��ʾ)����������Cuԭ����ĿΪ_____________________��
B.����ͪ��һ����Ҫ�Ļ���ԭ�ϣ�ʵ���ҳ������з����Ʊ�����ͪ��

������������ͪ��ˮ�IJ����������ʼ��±���
���� | �е�(��) | �ܶ�(g��cm-3�� | �ܽ��� |
������ | 161.1(97.8)* | 0.962 4 | ������ˮ |
����ͪ | 155.6(95)* | 0.947 8 | ����ˮ |
ˮ | 100.0 | 0.998 2 |
|
*�����е����ݱ�ʾ���л�����ˮ�γɵľ��й̶���ɵĻ����ķе�
(1)����Na2Cr2O7��Һ������������Ӧ�Ħ�H��0����Ӧ���ҽ�������ϵ�¶�Ѹ������������Ӧ���ࡣʵ���н�����Na2Cr2O7��Һ�ӵ�ʢ�л���������ƿ�У���55��
������Na2Cr2O7��Һ�ļ��Ϸ�ʽΪ_____________________��
�������ܷ��뻷��ͪ��ˮ��ԭ����_____________________��
(2)����ͪ���ᴿ��Ҫ��������һϵ�еIJ�����a.�����ռ�151��
��������������ȷ˳����_______________________(����ĸ)��
����������b��c��ʹ�õIJ����������ձ�����ƿ���������⣬����_______________��
������������c������NaCl�����������_______________________________________
______________________________________________________________________________��
(3)���ú˴Ź��������Լ����Ʊ��IJ����Ƿ�Ϊ����ͪ������ͪ��������______________�ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������С�⡣?
![]()
��.��ͼ��һ��ʵ��������װ�ã�ij����С������������װ�ÿ�����ȡ�������Ȼ������壬��ѡ�õ��Լ��У�A.ŨH2SO4��B.Ũ���ᣬC.ʳ�Σ�D.MnO2��?E.H2O2��aq��?��F.KClO3��G.KMnO4��Һ��������������⣺?
��1����Ҫ�����Ʊ�����������Ӧѡ��___________��ѡ���ţ���?
��2����Ҫ�����Ʊ�����HCl��Ӧѡ��___________��ѡ���ţ���?
��.��֪H2O2��aq�������ԣ�Ҳ��Ư�����ã�����MnO2�Ĵ��¿��Լ��ٷֽ⣺?
��2H2O2====2H2O+O2��?
ijѧ���������Ϻ�ָ��Na2O2��ˮ��Ӧʱ��������NaOH��H2O2��?
��Na2O2+2H2O====2NaOH+H2O2?
�˷�Ӧ�ų�����������ʹH2O2�����ֽ⣬����O2��?
��2H2O2====2H2O+O2��?
�ù��̵��ܷ�Ӧ����ʽ��Ϊ��?
��2Na2O2+2H2O====4NaOH+O2��?
��ͬѧ���ڵĿ�����ȤС������֤����������ʶ��ʵ�����ṩ������������?ҩƷ��??
�������Թܡ���ͷ�ιܡ��ձ���ҩ�ס�����?
ҩƷ��Na2O2��ˮ����̪��Һ��ʯ����Һ��MnO2?
�������Ǹÿ���С��ij�Ա���������ʵ����̣���д��ʵ�������ʵ��?���ۡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com