
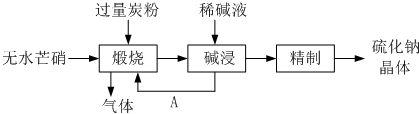
��â����Na2SO4 ��10H2O���Ʊ���������[��NH4��2Al��SO4��2��12H2O]������������������ͼ��

��1����ҺC�е�������Ҫ��_____________
��2������淋���Һ��_____�ԣ�����刺����ھ�ˮ�������ӷ���ʽ˵����ԭ��______________________
��3�����̢��еķ�Ӧ�¶Ȳ��ܳ���40�棬��ԭ����______________________________
��4�����û�ѧƽ��ԭ������Na2SO4�Թ�����ԭ��
��5������Al2��SO4��3���뵽A�л���������ij���������,��������淋IJ�������,�������ӷ���ʽ���Ͳ����������ԭ����___________________________________________
��6����ҺE�е���������Ϊ__________________
��7����֪����淋��ܽ�������¶ȵ����߶������̢��еõ�����淋�ϵ��ʵ�������: �� �����ˡ�ϴ�ӡ����
(1) NH4HCO3
��2���� Al3+ + 3H2O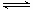 Al(OH)3
+ 3H+
Al(OH)3
+ 3H+
��3��NH4HCO3�����ȷֽ�
��4��HCO32����aq�� + Na+��aq��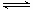 NaHCO3(s)��Na+Ũ������ƽ��������Ӧ������
NaHCO3(s)��Na+Ũ������ƽ��������Ӧ������
��5��Al3+ + 3HCO32�� =Al(OH)3�� + 3CO2��
��6��NH4+ ��SO42��
��7������Ũ������ȴ�ᾧ
��������
�������������Ϊ��ҵ�����⣬����һϵ�з�Ӧ���ᴿ���ᾧ�õ�����1��CO2�백ˮ��Ӧ����NH4HCO3��(NH4)2CO3����2������Al3+ ��NH4+ˮ��ʹ��Һ�������ԣ�����刺����ھ�ˮ������Ϊ������ˮ��������������������£�Al3+ + 3H2O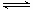 Al(OH)3
+ 3H+����3��NH4HCO3�����ȷֽ⣬�ʷ�ӦӦ�����¶ȣ���4����ƽ����ϵ������һ�ַ�Ӧ���Ũ�ȣ���Ӧ������У�������ⷴӦ���ת���ʣ���5��˫ˮ�ⷴӦ����6��AΪ(NH4)2SO4��ʣ��������κ�̼���Σ�B��ҪΪNH4HSO4��D��ҪΪʣ���NH4HSO4��Al2(SO4)3��EΪ(NH4)2SO4����7������淋��ܽ�������¶ȵ����߶����ʲ��ý��½ᾧ�ķ������룬����ҺŨ�ȵͣ�������ҪŨ��������Ϊ����Ũ�������½ᾧ����ȴ�ᾧ����
Al(OH)3
+ 3H+����3��NH4HCO3�����ȷֽ⣬�ʷ�ӦӦ�����¶ȣ���4����ƽ����ϵ������һ�ַ�Ӧ���Ũ�ȣ���Ӧ������У�������ⷴӦ���ת���ʣ���5��˫ˮ�ⷴӦ����6��AΪ(NH4)2SO4��ʣ��������κ�̼���Σ�B��ҪΪNH4HSO4��D��ҪΪʣ���NH4HSO4��Al2(SO4)3��EΪ(NH4)2SO4����7������淋��ܽ�������¶ȵ����߶����ʲ��ý��½ᾧ�ķ������룬����ҺŨ�ȵͣ�������ҪŨ��������Ϊ����Ũ�������½ᾧ����ȴ�ᾧ����
���㣺�Թ�ҵ����Ϊ��������������������ת���������Ŀ��ơ�����������й����⡣


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
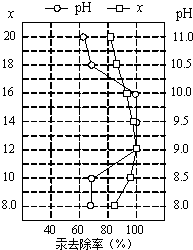 ��4����Ƥ�﹤ҵ��ˮ�еĹ��������Ƴ�ȥ������ȥ��������Һ��pH��x��x�������Ƶ�ʵ�����������������ı�ֵ���йأ���ͼ��ʾ����Ϊʹ����Ч����ѣ�Ӧ���Ƶ�������
��4����Ƥ�﹤ҵ��ˮ�еĹ��������Ƴ�ȥ������ȥ��������Һ��pH��x��x�������Ƶ�ʵ�����������������ı�ֵ���йأ���ͼ��ʾ����Ϊʹ����Ч����ѣ�Ӧ���Ƶ��������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com