
| ����������Һ ����ʽ��NaOH ��Է���������40 �ܶȣ�1.2g?cm-3 ����������20%��1����NaOH��Һ�����ʵ���Ũ��Ϊ 6 6 mol/L����2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� 24.0 24.0 g�����������ƣ���Һ���Ƶ�����Ļ����������£� ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� CBDFAE CBDFAE ����4������ʵ�鲽��A��B��E��F���õ�����������Ϊ 100ml����ƿ 100ml����ƿ ����5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ ƫ�� ƫ�� ��������ƿ��ԭ����������ˮ ��Ӱ�� ��Ӱ�� ���۶���ʱ���ӹ۲�Һ�� ƫ�� ƫ�� ��
��������1������c=
��2������m=nM=cVM�������Ƹ�Ũ�ȵ�NaOH��Һ100ml��Ҫ�������Ƶ������� ��3��������Һ���ƵIJ����������������Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ� ��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ�� ��5�������������������ʵ�������Һ�����Ӱ�죬����c=
����⣺��1����NaOH��Һ�����ʵ���Ũ��Ϊ��
��2������6mol/L��NaOH��Һ100ml��Ҫ�������Ƶ�����Ϊ��0.1L��6mol/L��40g/mol=24.0g���ʴ�Ϊ��24.0�� ��3����Һ���Ʋ��裺������������ܽ����ȴ��ת�ơ�ϴ�ӡ����ݡ�ҡ�ȣ�����Һ����ʵ������Ⱥ��������Ϊ��CBDFAE���ʴ�Ϊ��CBDFAE�� ��4����ͼ��֪������ʵ�鲽��A��B��E��F���õ�����������Ϊ100ml����ƿ���ʴ�Ϊ��100ml����ƿ�� ��5����ҡ�Ⱥ���Һ����ڿ̶��ߣ�������Һ������ƿ����ƿ��֮�䣬�ټ�ˮ���̶��ߣ�����������Һ�����ƫ��������Һ��Ũ��ƫ�ͣ� �ʴ�Ϊ��ƫ�ͣ� ����Һ�������ˮ���ݣ�����ƿ��ԭ����������ˮ����������Һ��Ӱ�죬�ʴ�Ϊ����Ӱ�죻 �۶���ʱ���ӹ۲�Һ�棬����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ� ���������⿼�����ʵ���Ũ�ȼ��㡢�ƶ����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע�����c=
 
��ϰ��ϵ�д�
 �ƸԾ���Ȥζ����ϵ�д� �ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д� ����С����ҵ��ϵ�д�
���ϰ��
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ʵ�飺 ��1����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺ 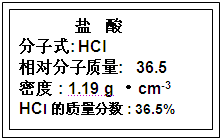 �ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ 11.9 11.9 mol?L-1����ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����BD BD ��A����Һ��HCl�����ʵ��� B����Һ��Ũ�� C����Һ��Cl-����Ŀ D��Һ���ܶ� ��2��ʵ��������480mL0.08mol/LNa2CO3��Һ�ش��������� ��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� 11.4 11.4 g�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ƽƽ��ʱ����ʵ�ʳ�����̼���ƾ����� 10.6 10.6 g��1g���������룩��������ƿ����һ�����ʵ���Ũ�ȵ���Һ��������ƿ������ B B A������ġ���B��ƿ����©ˮ��C���������Ƶ���Һ��ϴ������D���������Ҫ�� ����ʵ���������������Һ��Ũ���ǡ�ƫ�ߡ�����ƫ�͡����ǡ����䡱�� A����ˮʱԽ���̶��� ƫ�� ƫ�� ��B�����ǽ�ϴ��Һ��������ƿ ƫ�� ƫ�� ��C������ƿ�ڱڸ���ˮ���δ���ﴦ�� ���� ���� ��D���ܽ��û����ȴ����ж��� ƫ�� ƫ�� ����3����ȡ����Fe2O3��ĩ������ɫ�������������ᣬ��Ӧ�����ӷ���ʽΪ Fe2O3+6H+=2Fe3++3H2O Fe2O3+6H+=2Fe3++3H2O ����Ӧ��õ���ɫ��FeCl3��Һ���ô���Һ������ʵ�飺��ȡ������Һ�����Թ��У�����NaOH��Һ�������к��ɫ�������ɣ���Ӧ�����ӷ���ʽΪ Fe3++3O H-=Fe��OH��3�� Fe3++3O H-=Fe��OH��3�� ������С�ձ��м���25mL����ˮ�����������ں����ˮ�м���2mL FeCl3������Һ�������������Һ�� ��� ��� ɫ�������Ƶ�Fe��OH��3���壮����ȡһС�ձ�����25mL����ˮ�����ձ����ټ���2mL FeCl3������Һ�����Ⱥ����ձ�����żף���ʢ��Fe��OH��3������ձ�������ң�һ����ð������ֱ��ü���������ձ��е�Һ�壬���Կ��� �� �� ������ң��ձ��л���������ЧӦ����ʵ�����������Һ�ͽ��� ��Һ�ͽ��� ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ������ ��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml�������______g�����������ƣ���Һ���Ƶ�����Ļ����������£� 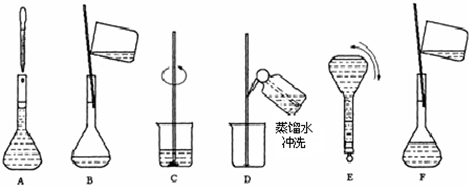 ��3��������ʵ�鲽��A��F��ʵ������Ⱥ��������______�� ��4������ʵ�鲽��A��B��E��F���õ�����������Ϊ______�� ��5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ______�� ������ƿ��ԭ����������ˮ______�� �۶���ʱ���ӹ۲�Һ��______�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ������ ���ͣ������ ��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml�������______g�����������ƣ���Һ���Ƶ�����Ļ����������£� 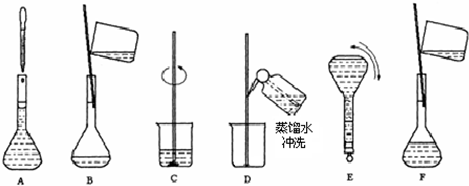 ��3��������ʵ�鲽��A��F��ʵ������Ⱥ��������______�� ��4������ʵ�鲽��A��B��E��F���õ�����������Ϊ______�� ��5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ______�� ������ƿ��ԭ����������ˮ______�� �۶���ʱ���ӹ۲�Һ��______�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ���أ��У�һ��������һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������ ��ͼΪʵ����ij�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��2������Ҫ���Ƹ�Ũ�ȵ�NaOH��Һ100ml������� g�����������ƣ���Һ���Ƶ�����Ļ����������£� 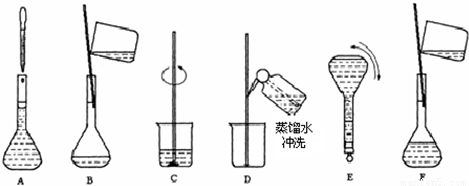 ��3��������ʵ�鲽��A��F��ʵ������Ⱥ�������� �� ��4������ʵ�鲽��A��B��E��F���õ�����������Ϊ �� ��5�����в�����NaOH��Һ�����ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��ҡ�Ⱥ���Һ����ڿ̶����ټ�ˮ �� ������ƿ��ԭ����������ˮ �� �۶���ʱ���ӹ۲�Һ�� �� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� | |||||||||||||||||||||||||||||||