
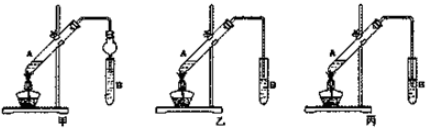
���� ��1������ȡ�����������˷�Ӧ���Ҵ��������⣬����Ũ��������������ˮ����������Һ�ܶȴ�С�жϼ����Լ���˳��
�ڳ����ܲ����쵽B�Թ�Һ���£���ֹ�����Һ��������ȷ�Ӧ����Թ��У�
���Ʊ���������ʱ���ñ���̼������Һ����������������Ҫ���������������������ڱ���̼���ƣ��������������ܽ�ȣ����ڷֲ㣬������ȡ��Һ�ķ������������Ҵ���ˮ���ܣ������ܱ�̼�������գ����ڳ�ȥ���ʣ�
��2����װ��ͼ������֪�������Ƭ�Ǵ����ã����Ƭ�д��ͻ����������ÿ�ȼ�������ȼ��Ҫ�������崿�ȣ�
�ڲ������������������Һ������ɫ��
�����ø��������Һ����ϩ����������ȼ�չ۲����������ƣ�
��� �⣺��1������ȡ����������Ũ������������A�Թ��е�Һ̬���������ᡢ�Ҵ���Ũ���ᣬ�����Լ�Ӧ�ȼ����ܶ�С���ټ����ܶȴ���Լ�����A�Թ��л���Ҵ���Ũ����IJ����ǣ������Թ�A�м����Ҵ���Ȼ���������Թ�ע��Ũ���ᣬ�ӱ����Թܣ�
�ʴ�Ϊ�������Թ�A�м����Ҵ���Ȼ���������Թ�ע��Ũ���ᣬ�ӱ����Թܣ�
�ڳ����ܲ����쵽B�Թ�Һ���£���ֹB�Թ�����Һ������A�Թ��У���װ��B�Թ��е�������Һ���£�������������
�ʴ�Ϊ������
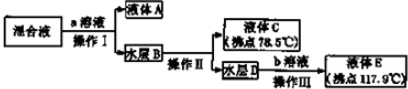
�۲�������Ҫ�����Һת�Ƶ���Һ©������ȡ��Һ��������������õ��Ҵ���ˮ��Ϊ�����ƣ�����������Һ��Һ�õ����ᣬ����õ����ᣬ
�ʴ�Ϊ����Һ©�����������
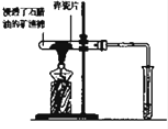
��2���ټ���ʯ����ʱ�������Ƭ��ʯ���ͷֽ�ϻ������������Ƭ�ܼӿ췴Ӧ���ʣ����Ƭ�����������������������Ӷ��ٽ�ʯ���ͷֽ⣬�������ã���ȼ����ǰ������еIJ����Ǽ������崿�ȣ�
�ʴ�Ϊ���������������崿�ȣ�
�ڲ������������������Һ������ɫ������������ʹ����ɫ��
�ʴ�Ϊ������������
��ʯ���ͷֽ�IJ����к���ϩ������������֤�����ɵ������к���������ʵ�����Ϊ�������ɵ�����ͨ��ʢ�����Ը�����ص���Һ��ϴ��ƿ���ȼ�����������Ļ����ҷų��������ȣ�
�ʴ�Ϊ�������ɵ�����ͨ��ʢ�����Ը�����ص���Һ��ϴ��ƿ���ȼ�����������Ļ����ҷų��������ȣ�
���� ���⿼����������������ȡ����Ŀ�Ѷ��еȣ�ע������������������ȡԭ����װ��ѡ����ȷ��Ӧ�б���̼������Һ�����ü��������������ĵ��ܵ���ȷ����������


 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
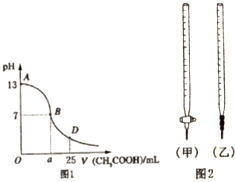
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ��̪ | ���ף� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ʯ�� | ���ң� |
| D | �� | �� | ʯ�� | ���ң� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������ڹ��յ�����������һ�ȼ���ķ�Ӧ | |
| B�� | ��ϩ��������Ȼ�̼��Һ����������ķ�Ӧ | |
| C�� | ��ϩ��ˮ�����Ҵ��ķ�Ӧ | |
| D�� | ��ϩ�������ɾ���ϩ�ķ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
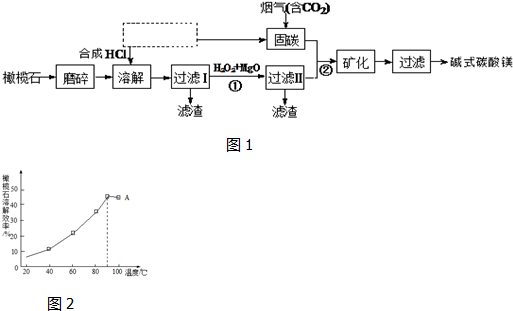
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�Ľṹ��ʽCH2CH2 | B�� | Na2O2����Ԫ�صĻ��ϼ�Ϊ-2 | ||
| C�� | Cl-�Ľṹʾ��ͼ�� | D�� | ��������ʽ��CH2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
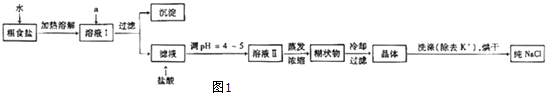
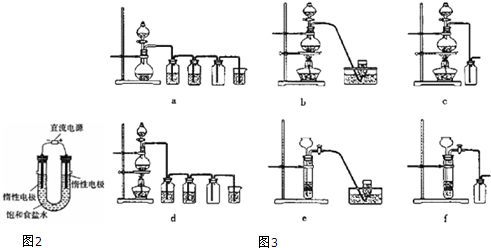
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��Ӧ�ﵽ��ѧƽ��״̬�����淴Ӧ������ȣ���ָͬһ���ʵ��������ʺ�����������ȣ����ò�ͬ���ʱ�ʾʱ����Ӧ���ʲ�һ����� | |
| B�� | ��״���£�1L������ȫȼ������CO28L | |
| C�� | 2.4gMg������O2������N2��ȫ��Ӧ��ת�Ƶ���������0.2NA | |
| D�� | 1L 1mol•L-1 CH3COOH��Һ�У�����CH3COO-��CH3COOH������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� | �� | �� | ���������� | ���������� | |
| ��һ�� | Na2CO3 | H2SO4 | NaHCO3 | CaO | CO2 |
| �ڶ��� | NaOH | HCl | NaCl | Na2O | CO |
| ������ | NaOH | CH3COOH | CaF2 | Al2O3 | SO2 |
| ���� | M | N | Q | P |
| ��Ӧǰ������g�� | 50 | 1 | 3 | 12 |
| ��Ӧ��������g�� | X | 26 | 3 | 30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��S��0 | B�� | ��S��0 | C�� | ��S=0 | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com