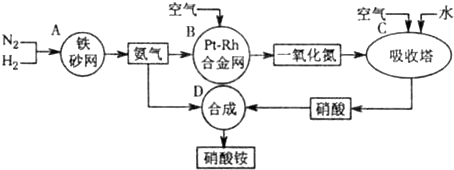
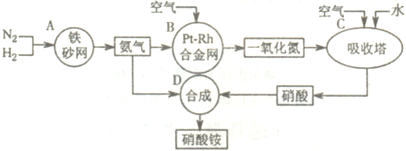
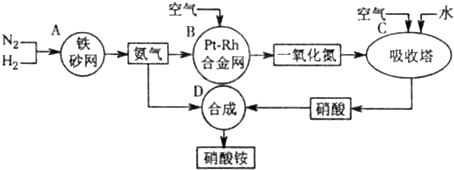
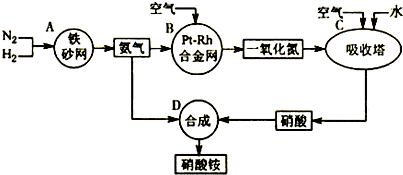
��2011?����ģ�⣩��ͼ�ǹ�ҵ��������淋�����ʾ��ͼ��
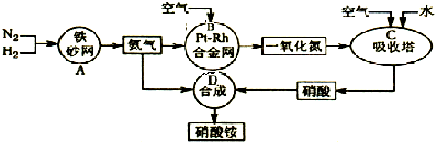
��1��������C��ͨ�����������Ŀ����
ʹNO������������ߡ����NO��ת���ʡ���
ʹNO������������ߡ����NO��ת���ʡ���
��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ����
ABC
ABC
������ĸ����
��2����֪��4NH
3��g��+3O
2��g���T2N
2��g��+6H
2O��g������H=-1266.8kJ/mol��
N
2��g��+O
2��g���T2NO��g������H=+180.5kJ/mol
�ݴ�д�������´��������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g��=4NO��g��+6H2O��g������H=-905.8KJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g������H=-905.8KJ/mol
��
����������������ת����5mol���ӣ���Ӧ�������仯Ϊ
226.45
226.45
kJ��
��3���ڻ����о��У�����Ҫ�жϷ�Ӧ�ܷ��Է����У���ij��Ӧ�ġ�H��0����÷�Ӧ�Ƿ�һ�����Է����У�
��һ��
��һ��
���һ������һ������
��4����֪��N
2��g��+3H
2��g��?2NH
3��g������H=-92kJ/mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��
CDE
CDE
������ĸ����
A���ʵ������¶�
B��ʹ�ø���Ч�Ĵ���
C������ѹǿ
D��ѭ�����úͲ��ϲ��䵪��
E����ʱ���������
��5����һ���¶Ⱥ�ѹǿ�£���H
2��N
2��3��1����������ܱ������л�ϣ�����Ӧ�ﵽƽ��ʱ�����ƽ��������NH
3���������Ϊ20.0%����ʱH
2��ת����Ϊ
33.3%
33.3%
������������һλС������



 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�