
 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
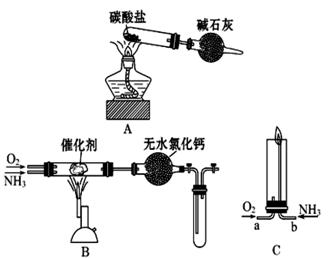
 Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺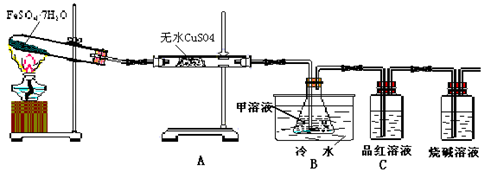
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
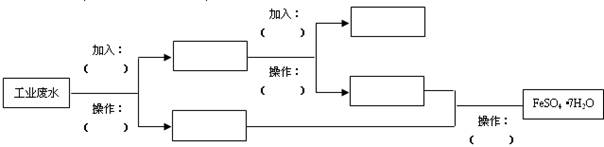
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
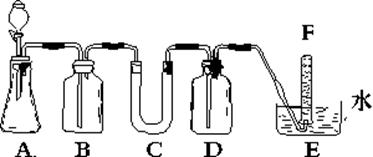
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��B�е������в������� | B��B�е������� �γ�һ��ˮ�� �γ�һ��ˮ�� |
| C������Ƭ���ڱ�� | D��п����ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ���������зֱ�ŵ�������ֽ����ȡ2.0g NaOH���� |
| B����NaOH�������ձ����ܽ��Ѹ��С��ת����250mL����ƿ�� |
| C������ʱ��С�ļ�ˮ�����˿̶��ߣ���ʱѸ���ý�ͷ�ι�����һЩ |
| D����Һ������ϣ���������ת�������������Լ�ƿ�����ϱ�ǩ��ϴ������ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ��ˮ����������Һ�� | ʵ������ | ���� |
| A | ����KSCN��FeCl2��Һ | ��� | Cl2���л�ԭ�� |
| B | ���з�̪��NaOH��Һ | ��ɫ | Cl2�������� |
| C | ��ɫʯ����Һ | �ȱ�����ɫ | Cl2����Ư���� |
| D | KI������Һ | ����ɫ | Cl2���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ô���Һ��ϴ�ζ��õ���ƿ |
| B���к͵ζ�ʱ���ֲ����ζ��ܣ�����ҡ����ƿ���۾�ע�ӵζ���Һ��ı仯 |
| C������ʽ�ζ�����ȡ20.00 mL KMnO4��Һ |
| D��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������ �����������ۣ���������ȩ�� �����������ۣ���������ȩ�� |
B�� ij±�������� ij±�������� ���յij������ǰ�ɫ�� ���յij������ǰ�ɫ�����ۣ���±�����в�����ԭ�� |
C��ij ��Һ ��Һ ð�Ű��� ð�Ű��� �����������̣� �����������̣����ۣ�����ҺΪŨ���� |
D����ɫ��Һ �ʻ�ɫ�����ۣ�����Һһ��������Ԫ�� �ʻ�ɫ�����ۣ�����Һһ��������Ԫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com