
��13�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣
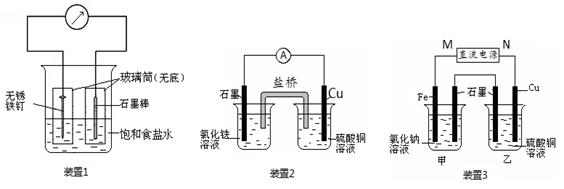
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ�������������IJ���Ͳ�ڵ���K3[Fe(CN)6] ��Һ�����ɹ۲쵽������������Һ����ɫ�������������� ��� ��������ԭ������ �����̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪��Һ���۲쵽ʯī�缫�������ȱ�졣
�� ��Դ��M��Ϊ ��������������������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�Ļ�ѧ����ʽΪ ��
�� ֹͣ��⣬ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0.64 g�����ձ��в����������״�������Ϊ mL ��
��13�֣���1��������1�֣��� O2+ 4e��+ 2H2O = 4OH����2�֣�
��2������1�֣��� 2Fe3�� + Cu = 2Fe2�� + Cu2�� ��2�֣�
��3���� ����1�֣��� Fe -2e��= Fe2����2�֣�
�� 2CuSO4+ 2H2O 2Cu + O2�� + 2H2SO4 ��2�֣� �� 224 ��2�֣�
2Cu + O2�� + 2H2SO4 ��2�֣� �� 224 ��2�֣�
��������
�����������1��װ��1Ϊ���ĵ绯ѧ��ʴ��������������Һ����ɫ����������������������Ϊ������̼Ϊ�������۲쵽̼����������Һ��죬˵����̼���������õ�������OH-���ӣ���Ӧ�ĵ缫��ӦʽΪO2+ 4e��+ 2H2O = 4OH����
��2��װ��2Ϊԭ��أ�����ΪCu��ͭʧȥ���ӣ�����ͭ���Ӷ��ܽ⡣ʯī����������Һ�е������ӵõ����������������ӣ������ܵ����ӷ���ʽ��2Fe3�� + Cu = 2Fe2�� + Cu2����
��3���ٷ�Ӧһ��ʱ���ֹͣͨ�磮����ձ��е��뼸�η�̪���۲쵽ʯī�缫�������ȱ�죬˵����ʯī�缫������OH-���ӣ���ʯī����������������������M�ǵ�Դ�������������缫�ķ���ʽ��Fe -2e��= Fe2����
�����ձ��������ͭ��Һ��ʯīΪ������ͭ�����������Ե����ܷ���ʽ��2CuSO4��2H2O 2Cu �� O2���� 2H2SO4��
2Cu �� O2���� 2H2SO4��
��ȡ��Cu�缫��ϴ�ӡ�����������缫����0.64g��������Cu�����ʵ���Ϊ0.64g ��64g/mol ��0.01mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.01mol��2��0.02mol
���ݼ��ձ���������ĵ缫��Ӧ��֪
2H��+2e����H2��
2mol 22.4L
0.02mol V
����V��0.224L��
���㣺����绯ѧԭ�����ۺ�Ӧ�á������ĸ�ʴ�ͷ������缫��Ӧʽ����д���缫���Ƶ��ж��Լ��йؼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ���������У����ؿ���ѧ���������⡢������������������ʱҪע��缫���жϺ͵缫��Ӧ����д��ע�����·�и��缫ת�Ƶĵ�����Ŀ��ȣ����÷�Ӧ�ķ���ʽ���㼴�ɡ�


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0��2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0��5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0��64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0.64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�ϰ����ȹ���ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⡣
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ�����Һ����dz��ɫ���������� �����������ԭ�����������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ ��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���Ϊ ��
��3��װ��3�м��ձ�ʢ��100 mL 0.2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0.5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣����ձ��е��뼸�η�̪���۲쵽ʯī�缫�������ȱ�졣
�ٵ�Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�����ձ��е�ⷴӦ�����ӷ���ʽΪ ��
��ֹͣ��⣬ȡ��Cu�缫��ϴ�ӡ�����������缫����0.64 g�����ձ��в����������״�������Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������Ĵ��¿���ѧ�Ծ� ���ͣ������
��12�֣���ͼ��ʾ3��ʵ��װ�ã��ֱ�ش��������⣺
 ��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��1��װ��1Ϊ����������ʴʵ�顣һ��ʱ��������̼���IJ���Ͳ�ڵ����̪��Һ���ɹ۲쵽̼����������Һ��죬�õ缫��ӦΪ
��
��2��װ��2�е�ʯī�� �������������������װ�÷������ܷ�Ӧ�����ӷ���ʽΪ ��
��3��װ��3�м��ձ�ʢ��100 mL 0��2 mol/L��NaCl��Һ�����ձ�ʢ��100 mL 0��5 mol/L��CuSO4��Һ����Ӧһ��ʱ���ֹͣͨ�硣ȡ��Cu�缫��ϴ�ӡ�����������缫���� 0��64 g��
�� ��Դ��M��Ϊ �������ձ������缫�ĵ缫��ӦΪ ��
�� ���ձ��е�ⷴӦ�����ӷ���ʽΪ ��
�� �ס������ձ������ɵ������״���¹� mL��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com