
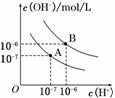
 ��֪ˮ��25���95��ʱ�������ƽ����������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ����������ͼ��ʾ��
(1)��25��ʱˮ�ĵ���ƽ������ӦΪ________(�A����B��)����˵������__ ______________________________________________
______________________________________________
_____________________________________________________��
(2)25��ʱ����pH��9��NaOH��Һ��pH��4��H2SO4��Һ��ϣ������û����Һ��pH��7����NaOH��Һ��pH��4��H2SO4��Һ�������Ϊ__________��
(3)95��ʱ����100���pH1��a��ijǿ����Һ��1���pH2��b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2 ֮��Ӧ����Ĺ�ϵ��____________��
֮��Ӧ����Ĺ�ϵ��____________��
(4)����B��Ӧ�¶��£�pH��2��ijHA��Һ��pH��10��NaOH��Һ�������Ϻ����Һ��pH��5���������ԭ��____ __________________________________
__________________________________
________________________________________________________________________��
����������Ĺؼ��Ǹ�����¶ȶ�ˮ�ĵ���ƽ�⡢ˮ�����ӻ�����ҺpH��Ӱ�졣
(1)���¶�����ʱ���ٽ�ˮ�ĵ��룬ˮ�����ӻ�Ҳ����ˮ��������Ũ�ȡ�����������Ũ�ȶ�����ˮ��pH��С������Һ��Ȼ�����ԡ���˽��ͼ����A��B���߱仯�����������Ũ�ȡ�����������Ũ�ȿ����жϣ�25��ʱˮ�ĵ���ƽ������ӦΪA������Ϊˮ�ĵ��������ȹ��̣������¶ȣ�ˮ�ĵ���̶�����
(2)25��ʱ���û����Һ��pH��7����Һ�����Լ����ǡ���кͣ���n(OH��)��n(H��)����V(NaOH)��10��5 mol/L��V(H2SO4)��10��4 mol/L����V(NaOH)��V(H2SO4)��10��1��
(3)Ҫע����95��ʱ��ˮ�����ӻ�Ϊ1��10��12����c(H��)��c(OH��)��1��10��12��������ǿ�ᡢǿ�Ӧ������ʱpH(��)��pH(��)��12������95��ʱ��Ϻ���Һ�����ԣ�pH2��b��ijǿ����Һ��c(OH��)��10b��12����100��10��a��1��10b��12���ɵ�10��a��2��10b��12�����ԣ������¹�ϵ��a��b��14��pH1��pH2��14��
(4)������B��Ӧ�¶��£���pH(��)��pH(��)��12���ɵ��������Һ��c(H��)��c(OH��)������ǿ�ᡢǿ�����Һ�������Ϻ���ҺӦ�����ԣ��ֻ����Һ��pH��5�����������Ϻ���Һ�����ԣ�˵��H����OH����ȫ��Ӧ�������µ�H���������������������˵��HA�����ᡣ
�𰸣�(1)A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ������̶�С��c(H��)��c(OH��)С
(2)10��1��(3)a��b��14��pH1��pH2��14
(4)����B��Ӧ95�棬��ʱˮ�����ӻ�Ϊ1��10��12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H����ʹ��ҺpH��5


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й���������˵��M��N�ķǽ�����ǿ��������
�ٷǽ�������M�ܴ�N�Ļ��������û����ǽ�������N��
��Mԭ�ӱ�Nԭ�����õ����ӡ�
�۵���M��H2��Ӧ��N��H2��Ӧ���öࡣ
����̬�⻯��ˮ��Һ������HmM��HnN��
������������Ӧˮ���������HmMOx��HnNOy��
���۵�M��N��
A.�٢ڢۢ� B.�ڢ�
C.�٢ڢۢܢ� D.ȫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���·�Ӧ�������ɫ��ѧԭ�Ӿ�����Ҫ�����(����)
A����ϩ�ۺ�Ϊ����ϩ�߷��Ӳ���
B�������������Ʊ�һ�ȼ���
C����ͭ��Ũ����Ϊԭ����������ͭ
D����SiO2�Ʊ��ߴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��ѡ�������ָʾ��������д���пհף�
(1)�ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��___________________________________________________��
ֱ�������һ���������Һ�ɻ�ɫ��Ϊ��ɫ����________________Ϊֹ��
(2)���в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���________��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
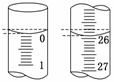 D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
(3)���ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ________mL���յ����Ϊ__________mL��
����������Һ�����Ϊ______mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A�������Ǵ�ˮ���������ԡ����Ի��� ��ϡ��Һ���ڳ����£���c(H��)��c(OH��)��1��10��14
��ϡ��Һ���ڳ����£���c(H��)��c(OH��)��1��10��14
B��c(H��)����1��10��7 mol/L����Һ��һ����������Һ
C��0.2 mol/L CH3COOH��Һ�е�c(H��)��0.1 mol/L CH3COOH��Һ�е�c(H��)��2��
D���κ�Ũ�ȵ���Һ��������pH����ʾ�����Ե�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪1 g������ȫȼ������ˮ����ʱ�ų�����121 kJ����������1 mol O=O����ȫ�� ��ʱ��������496 kJ��������1 mol H�DH������ʱ��������Ϊ436 kJ����ˮ������1 mol H�DO���γ�ʱ�ų����� ( )
A�� 463kJ B�� 557 kJ C�� 486kJ D��188 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ȼ����ؿ���������������Ĺ�����Դ��һ���ȼ����صĻ���
�ṹ��ͼ��ʾ��������Ϊ����ʵ���ˮLiCl-KCl������������ں�ؼ���˲��������ܡ��õ���ܷ�ӦΪ��PbSO4+2LiCl+Ca ��CaCl2+Li2SO4+Pb�������й�˵����ȷ����: (��֪��Pb��ԭ���� 207 ) ( )
A��������Ӧʽ��Ca+2Cl- - 2e- ��CaCl2
B���ŵ�����У�Li�� ���ƶ�
C��ÿת��0.1mol���ӣ�����������20.7gPb
D������ʱ��������������ϵ�����������ƣ�ָ�벻ƫת
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ij�ֹ��ԣ��ɽ�SO3��CO2��Ϊͬ�����������������Ҳ����������������ǣ� ��
A��CaCO3 B��SO2 C��KMnO4 D��Na2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����
A����Ũ���ᱣ������ɫ����ƿ��
B�������ƺͼر�����ú����
C��Na2CO3���Ա����ڲ������IJ���ƿ��
D��NaOH���������ֽ�ϳ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com