
�黯�ڣ�GaAs���������İ뵼�����,�����������ͼ�������̫���ܵ�صIJ��ϵȡ��ش��������⣺
(1)��̬Gaԭ�ӵĺ�������Ų�ʽΪ[Ar]_______________��
(2)����Ԫ�������ɣ�Ԫ�صĵ縺��Ga______������ڡ���С�ڡ�, ��ͬ��As����һ������B ____ Ga��BF3��NH3�ķ����ܹ�ͨ����λ�����ϵ�ԭ����_______��
(3)ɱ���Na3AsO4�������ӵĿ��ʹ���Ϊ______��Asԭ�Ӳ�ȡ________�ӻ���
(4)������Ƶ�GaF3��GaCl3���壬ǰ���������Ӿ��壬�������ڷ��Ӿ��塣��F-��Cl-�ṹ�IJ�ͬ������ԭ����____________��
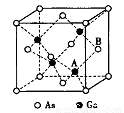
(5)ԭ�Ӿ���GaAs�ľ�������a=xpm�����ľ����ṹ����ͼ��ʾ���þ����ڲ����ڵĹ��۽���Ϊ______��Aԭ�Ӿ���Bԭ������������IJ������̾���Ϊ______ ����x��ʾ��pm ���þ������ܶ�Ϊ_____g��cm-3��������٤��������NA��ʾ����
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡ�߶���ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Һ�к�����������NaCl��H2SO4�����ǵ����ʵ���֮��Ϊ3��1�����뼸��ʯ����Һ����ʯī���缫���û����Һ�����ݵ缫��������̿����Է�Ϊ�����Ρ����������У�����ȷ����
A. ������ʼ����ֻ����H2
B. �������У���Һ��ɫ���ֺ�ɫ����ɫ����ɫ�ı仯
C. �������У�Na+��SO2- 4�����ʵ���Ũ�ȱ��ֲ���
D. ��һ����������������������������ȼ��ǡ����ȫ��Ӧ�õ�HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���ѧ����ĩ���ԣ�������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£����и���������ָ����Һ���ܴ����������
A. pH��1����Һ�У�Fe2����NO3����Na����SO42��
B. c(H��)/c(OH��)��1012��ˮ��Һ�У�NH4����Al3����Cl����NO3��
C. ˮ�������c(H��)��10��12 mol/L����Һ�У�Ca2����K����Cl����HCO3��
D. c(Fe3��)��0.1 mol/L����Һ�У�K����ClO����SO42����SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������и�����Ӧ���¿����壩���ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
��1������Ԫ���е�һ��������С����_____________����Ԫ�ط��ţ���ͬ��
��2����Ѫ������A��B��C��D����Ԫ���γɵ���λ������C4[D(AB)6]��������ˮ���㷺����ʳ�����Ӽ��������������д����Ѫ�εĻ�ѧʽ___ _��1molABһ�к��Цм�����ĿΪ__ ______(�����ӵ�������ֵΪNA)����Ѫ�ξ����и�����������������漰____ ������ţ���
a ���������� b�����ۼ� c����λ�� d�����Ӽ� e����� f�����Ӽ��������
��3��E2+�ļ۲�����Ų�ͼΪ ���ܶ�����л�����E���¿���H2�����ӳɷ�Ӧ����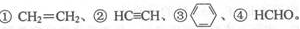 ����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� ����������ţ���HCHO���ӵ�����ṹΪ �����ӳɺ����״����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���� ��
����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� ����������ţ���HCHO���ӵ�����ṹΪ �����ӳɺ����״����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���� ��
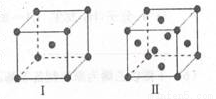
��4������C��F����ľ����ṹ��ͼ�������ж϶�Ӧ��ͼ����C��F���־��徧���н���ԭ�ӵ���λ��֮��Ϊ_ ___ ������C�ľ����У�����þ������ܶ�Ϊag/cm3�������ӵ�������ֵΪNA��Cԭ�ӵ�Ħ������ΪM g/mol�����ʾCԭ�Ӱ뾶�ļ���ʽΪ cm(���ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������и�����Ӧ���¿����壩���ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
������Ԫ��X��Y��Z��W��R��ԭ��������������Yԭ�Ӵﵽ�ȶ��ṹ��õĵ�����Ŀ�������ڲ������Ŀ��ȣ�X��Zͬ���壬Z��������������Ԫ����ԭ�Ӱ뾶����Ԫ�أ�W����������������Ӳ�����ͬ��R��Z�γɵĻ�������ˮ��Һ�ʼ��ԡ�����˵����ȷ����
A. ���Ӱ뾶�ɴ�С��˳��ΪR��Z��Y��W
B. X��Y�ֱ���Z�γɵĶ��ֻ������л�ѧ��������ͬ
C. Y��R�ֱ���X�γɵĻ�����е���������
D. Z��W��R����������Ӧ��ˮ��������֮����ܷ�����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����3�¸߿���Ӧ�Բ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������[CO(NH2)2]�ļ�����Ҵ������ȡ��������װ������ͼ��ʾ�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫���������й�˵���в���ȷ����
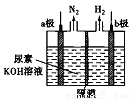
A. ��������b��������Һ����������ǿ
B. ��Һ�е�OH-����a���������ƶ�
C. ����b��������״����224mL����������������2g
D. a����ӦʽΪCO(NH2)2+8OH--6e-=CO32-+N2��+6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ������
��һ�������Ʊ���NaCl��Һ�������ձ��м��ȣ�
��һ���������¶���30-35 �档�߽���߷���������ϸ��NH4HCO3���壬������Ϻ�������30���ӣ�
�����������á����˵�NaHCO3���壮����������ˮϴ�ӳ�ȥ���ʣ�Ȼ���ɣ�
���IJ������ڶ������ù���ת���������У�����2Сʱ���Ƶô�����壬
��֪���¶ȸ���35��ʱ��NH4HCO3��ֽ⡣�й��ε��ܽ��(g/100gˮ)���±���
�� | 0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ���� | ���� | ���� | ���� |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.l | 12.7 | 14.5 | 16.4 | ���� |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
�ش��������⣺
(1)��Ӧ�¶ȿ�����30-35�淶Χ�ڣ�Ӧ��ȡ�ļ��ȷ���Ϊ ����Ӧ�¶Ȳ��ܸ���35��������� ��
(2)���������ú�����NaHCO3�����ԭ���� ��������ˮϴ��NaHCO3�����Ŀ���dz�ȥ�������� (�����ӷ��ű�ʾ)��
(3)�����������ĸҺ�м��� (��д�Լ�����)��������һ��������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4Cl��
(4)�����Ʒ����������NaHCO3��NaCl�����ʡ��ⶨ������NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷWg��������ƿ�м�����ˮ�ܽ⣬��l-2�η�ָ̪ʾ���������ʵ���Ũ��Ϊcmol/L������ζ�����Һ�ɺ�ɫ����ɫ(ָʾCO32-+H��=HCO3-��Ӧ���յ�), �����������ΪV1mL���ټ�1-2�μ���ָʾ��������������ζ�����Һ�ɻƱ�ȣ��������������ΪV2mL��
�� ʵ��������һ�����cmol/L�������õ�������������ƿ���ձ�����Ͳ��� ��������ƿ��ʹ�÷����У����в�����ȷ���� (��д���)
a��ʹ������ƿǰ�������Ƿ�©ˮ
b������ƿ������ˮϴ�������ô�����Һ��ϴ
c��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�Ȼ�������ˮ����
d��������ƿ����Һ��������Ժ�ֱ�����ϱ�ǩ�����ñ���
e�����ݺ�Ǻ�ƿ������ʳָ��סƿ��������һֻ��ָ��סƿ�ף�������ƿ��ת��ҡ������
�� д��������Ʒ��NaHCO3���������ļ���ʽ��NaHCO3(%)= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ�����ӵ�������ֵ������������ȷ����
A. 1.00mol NaCl���NA��NaCl ����
B. ���³�ѹ�£�22.4LCl2������þ�۷�Ӧ��ת�Ƶĵ�����Ϊ2NA
C. һ�������£�2.3gNa������O2��ȫ��Ӧ����3.6g����ʱʧȥ�ĵ�����Ϊ0.1NA
D. 28g��ϩ�ͻ�����(C3H6)��ɵĻ�������к�����ԭ�ӵĸ���Ϊ3 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijʳ�ð״����ɴ����봿ˮ���ƶ��ɣ����к͵ζ��ķ���ȷ�ⶨ���д�������ʵ���Ũ�ȡ�ʵ�鲽�裺������500mLŨ��ԼΪ0.1 mol��L��1��NaOH��Һ������KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��1�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⡣�����Ʋ��� ������С������С�����
��2������ʱNaOH�ڿ����м�����ˮ���������õ�NaOH��ҺŨ��ͨ����Ԥ�� ���С���������Dz���ֱ�����������Һ��ԭ��
��3�����İ״װ�װ˵�������Ậ��ԼΪ6g/100mL����������ʵ���Ũ��ԼΪ mol��L��1���ζ�ǰ���״�ϡ�� ���10����100������������֪�������Է�������Ϊ60��
��4��ϡ�Ͱ״�ʱ��Ҫ���������ձ������������ιܡ���ʽ�ζ��ܡ� ��
��5��ȷ��ȡϡ�ͺ�İ״�20.00mL������250mL��ƿ�У���ˮ30mL���ٵμӷ�ָ̪ʾ����������NaOH����Һ�ζ��� ��Ϊ�յ㡣�ظ��ζ����Σ�ƽ������NaOH����ҺV mL��NaOH��ҺŨ��Ϊc mol��L��1����
��6��ԭ�״��д�������ʵ���Ũ�ȣ� mol��L��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com