
��֪Ư����Ũ���ᷴӦ����������Ca(ClO)2 + 4HCl == CaCl2 + 2Cl2 + 2H2O��ijͬѧ��������װ���������ʵ�飺�ٴ��ԲⶨijƯ������Ч�ɷ�Ca(ClO)2�ĺ�������̽�����﴿����������Ư���ԣ���ʪ��������Ư���ԣ���Ư��������HClO�����á��ش��������⣺

��1��д����ҵ����ȡƯ�۵Ļ�ѧ����ʽ_________________________________
��2��������з�Ӧ����ת�Ƶķ������Ŀ����ָ����Ӧ�е��������ͻ�ԭ����
Ca(ClO)2 + 4HCl == CaCl2 + 2Cl2 + 2H2O
������_______________��ԭ��_________________
��3������ʵ��װ��B��Ӧ�ŵ��Լ���_____________��������_____________
��4��ijͬѧ��ij��ʵ��ʱ��ȡƯ����Ʒmg��ʵ��������ȡ��Ͳ��ˮ�����ΪVmL������ʵ��������DZ�״���������Ư������Ч�ɷֵĺ���____________________________���ú���ĸ�İٷ�����ʾ��
��5��ϸ�ĵ���ͬѧ˼�����ָ�ʵ���̽��ʵ����ۣ���һ�����ԵIJ��㣬�ò���֮���ǣ�����Ϊû�в��㣬�ÿղ���д��__________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
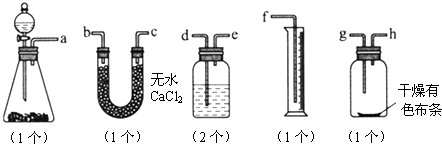
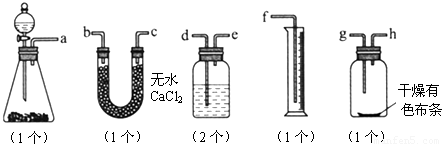
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 1.2��ѧʵ�����ɫ����ϰ���������棩 ���ͣ�ʵ����
��֪Ư����Ũ���ᷴӦ�ɲ�������[Ca(ClO)2��4HCl(Ũ)===CaCl2��2Cl2����2H2O]��ijͬѧ��ͼ�ⶨ�������������֤���﴿����������Ư���ԣ���������װ�ã���ش�
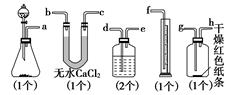
(1)������ĸ��дװ�õ�����˳��a��(����)��(����)��(����)��(����)��(����)��(����)��(����)��(����)��(����)��
(2)ϴ��ƿ����װҺ����________��
(3)��������ǰ��������е�һ�����������________________________________________________________________________��
(4)ijͬѧ��ʵ���У�������Ͳ��δ�ռ���Һ�壬��ʧ�ܵ�ԭ�������________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ����һ�и�һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com