
�ҹ�����ˮ��������������б���

������Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к�Ca2+��Mg2+��HCO3����Cl���ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ��________��
(2)���ۼ���ȥ������������Ĺ���________(��д���)��
��ֻ���������̡���ֻ�ǻ�ѧ���̡����������ͻ�ѧ����
FeSO4��7H2O�dz��õ����ۼ�������ˮ����������________������
(3)ͨ�������̼��Ŀ����________��________��
(4)����A��������________�����������ǻ�������A��ˮ��Ӧ�IJ������________�ԣ�
(5)���������У�________������Ϊ����A�Ĵ���Ʒ��(��д���)
��Ca(ClO)2������NH3(Һ)������K2FeO4������SO2
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��Ŀ | pH | Ca2+��Mg2+��Ũ��/mol?L-1 | ϸ������/mL-1 |
| ���ֵ | 6.5��8.5 | ��0.0045 | ��100 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿������С�����ѧ ���ͣ�022
�ҹ��涨����ˮ��������������б���

������Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к�Ca2+��Mg2+��![]() ��Cl���ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ��________��
��Cl���ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ��________��
(2)���ۼ���ȥ������������Ĺ���________(��д���)��
��ֻ���������̡�����ֻ�ǻ�ѧ���̡������������ͻ�ѧ����
FeSO4��7H2O�dz��õ����ۼ�������ˮ����������________������
(3)ͨ�������̼��Ŀ����________��________��
(4)����A��������________�����������ǻ�������A��ˮ��Ӧ�IJ������________�ԣ�
(5)���������У�________������Ϊ����A�Ĵ���Ʒ��(��д���)
��Ca(ClO)2������NH3(Һ)������K2FeO4������SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
�ҹ�����ˮ��������������б���

��Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к� ��
�� ��
��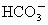 ��
�� �ȣ�����ʯ�Һ�����
�ȣ�����ʯ�Һ����� �������������ɸ��ֽⷴӦ��д�����е�һ�����ӷ���ʽ��
�������������ɸ��ֽⷴӦ��д�����е�һ�����ӷ���ʽ��
_____________________��
(2)���ۼ���ȥ������������Ĺ���________(��д���)����ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ���� �dz��õ����ۼ�������ˮ����������_________������
�dz��õ����ۼ�������ˮ����������_________������
(3)ͨ�������̼��Ŀ����___________��_____________��
(4)����A��������____________���������û�������A��ˮ��Ӧ�IJ������___________�ԣ�
(5)���������У�__________������Ϊ����A�Ĵ���Ʒ(��д���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
�ҹ�����ˮ��������������б���

��Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к� ��
��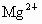 ��
�� ��
�� �ȣ�����ʯ�Һ�����
�ȣ�����ʯ�Һ����� �������������ɸ��ֽⷴӦ��д�����е�һ�����ӷ���ʽ��
�������������ɸ��ֽⷴӦ��д�����е�һ�����ӷ���ʽ��
_____________________��
(2)���ۼ���ȥ������������Ĺ���________(��д���)����ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ���� �dz��õ����ۼ�������ˮ����������_________������
�dz��õ����ۼ�������ˮ����������_________������
(3)ͨ�������̼��Ŀ����___________��_____________��
(4)����A��������____________���������û�������A��ˮ��Ӧ�IJ������___________�ԣ�
(5)���������У�__________������Ϊ����A�Ĵ���Ʒ(��д���)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com