
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011��߿���ѧ�ܸ�ϰ30������ʱѵ����ר��12����ѧ����Ȼ��Դ�Ŀ������� ���ͣ�058
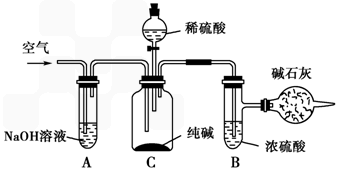
�����к��зḻ����Դ��������ľ⣮�ҹ�ӵ�кܳ��ĺ����ߣ������ذ���̲ƽ������ϸɳ������ʱ�䳤�������γ��������ڴˣ��ش��������⣺
(1)�ƺ��η������£���ˮ�dz�ʱ������ˮ����������䳱ʱ��������ɹ�ķ�����������ˮ��������һ���̶�ʱ��������ʳ�ο���(��Ϊ����)��ʳ�ξ��弴�ӱ�����Һ����������˵��������ʳ�ξ��������________��
(2)��ʳ��Ϊ��Ҫԭ�Ͽ����Ʊ�������û�ѧ����ʽ��ʾ���Ʊ����̣�
________��
�˷��ƵõĴ����г����������Ȼ��ƣ�Ҫ�ⶨ��Ʒ�еĴ�����������������ɲ�����ͼ�е�װ�ý���ʵ�飺
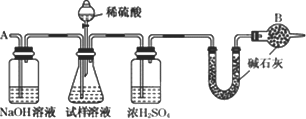
������һ�仰������ʵ����ƵĻ���ԭ����________��
(3)�ȼ�����Ƶõ��ռ���Ҳ���������Ȼ���(���費����������)�����к͵ζ������һ��ʵ�鷽���Բⶨ��Ʒ���ռ���Ȼ��Ƶ������������Լ���0.100 0 mol/L���ᡢ��̪��Һ��������50 mL����ʽ�ζ��ܡ���ƿ����ʵ�����Ҫ������________��________���ζ���
(4)��ҵ���õ�ⷨ�Ʊ������ƣ����Ʊ�������һ�㲻���õ�������Ȼ��أ����Dz����Ȼ�ԭ�����Ʊ�����850���ý���������ԭKCl��Ӧ���£�
Na(Һ)��KCl(Һ)![]() NaCl(Һ)��K(��)��
NaCl(Һ)��K(��)��
��ҵ��Ӧ������Щ��ʩ����ʹ��Ӧ������Ӧ�����ƶ���
________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
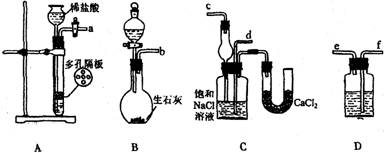
��14�֣��ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������NaHCO3��NaCl��NH4Cl�������ܽ�ȵIJ��죬��ʳ�Ρ�������������̼��Ϊԭ�����Ƶ�NaHCO3�����������������������ʵ������ģ�⡰�����Ƽ����ȡNaHCO3��ʵ�鲽�裺
��һ�������Ӻ�װ�ã����������ԣ���������װ��ҩƷ��
�ڶ���������һװ�÷�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������壬Ƭ�̺�C�г��ֹ��塣������C��ͨ���������壬ֱ�������й��������
������������C�����õĻ����õ�NaHCO3���塣
���IJ�������Һ�м���������NaCl��ĩ����NH4Cl��������������
��ش��������⣺
(1)װ�õ�����˳���ǣ�(a)��( ) ( )��( )��(b)��( )
(2)A�г�ѡ�õĹ��巴Ӧ��Ϊ____________��D��Ӧѡ�õ�Һ��Ϊ___________ ���ѧʽ��
(3)�ڶ������б�������________װ���ȷ�����Ӧ��
(4)C�������θ���ܶ�����ֱ���ܣ���������____________________________��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
(5)�ڵ��IJ��з���NH4Cl����IJ�����________________����������ƣ��������õ�NH4Cl�����г�����������NaCl��NaHCO3Լռ5%��8%���������һ����ʵ��֤�����ù���ijɷ��к���Na������Ҫд������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ�����ظ�����ģ�����������⣨��ѧ���֣� ���ͣ�ʵ����
��14�֣��ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������NaHCO3��NaCl��NH4Cl�������ܽ�ȵIJ��죬��ʳ�Ρ�������������̼��Ϊԭ�����Ƶ�NaHCO3�����������������������ʵ������ģ�⡰�����Ƽ����ȡNaHCO3��ʵ�鲽�裺
��һ�������Ӻ�װ�ã����������ԣ���������װ��ҩƷ��
�ڶ���������һװ�÷�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������壬Ƭ�̺�C�г��ֹ��塣������C��ͨ���������壬ֱ�������й��������
������������C�����õĻ����õ�NaHCO3���塣
���IJ�������Һ�м���������NaCl��ĩ����NH4Cl��������������
��ش��������⣺
(1)װ�õ�����˳���ǣ�(a)��( ) ( )��( )��(b)��( )
(2)A�г�ѡ�õĹ��巴Ӧ��Ϊ____________��D��Ӧѡ�õ�Һ��Ϊ___________ ���ѧʽ��
(3)�ڶ������б�������________װ���ȷ�����Ӧ��
(4)C�������θ���ܶ�����ֱ���ܣ���������____________________________��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
(5)�ڵ��IJ��з���NH4Cl����IJ�����________________����������ƣ��������õ�NH4Cl�����г�����������NaCl��NaHCO3Լռ5%��8%���������һ����ʵ��֤�����ù���ijɷ��к���Na������Ҫд������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ظ�����ģ�����������⣨��ѧ���֣� ���ͣ�ʵ����
��14�֣��ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������NaHCO3��NaCl��NH4Cl�������ܽ�ȵIJ��죬��ʳ�Ρ�������������̼��Ϊԭ�����Ƶ�NaHCO3�����������������������ʵ������ģ�⡰�����Ƽ����ȡNaHCO3��ʵ�鲽�裺
��һ�������Ӻ�װ�ã����������ԣ���������װ��ҩƷ��
�ڶ���������һװ�÷�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������壬Ƭ�̺�C�г��ֹ��塣������C��ͨ���������壬ֱ�������й��������
������������C�����õĻ����õ�NaHCO3���塣
���IJ�������Һ�м���������NaCl��ĩ����NH4Cl��������������
��ش��������⣺
(1)װ�õ�����˳���ǣ�(a)��( ) ( )��( )��(b)��( )
(2)A�г�ѡ�õĹ��巴Ӧ��Ϊ____________��D��Ӧѡ�õ�Һ��Ϊ___________ ���ѧʽ��
(3)�ڶ������б�������________װ���ȷ�����Ӧ��
(4)C�������θ���ܶ�����ֱ���ܣ���������____________________________��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
(5)�ڵ��IJ��з���NH4Cl����IJ�����________________����������ƣ��������õ�NH4Cl�����г�����������NaCl��NaHCO3Լռ5%��8%���������һ����ʵ��֤�����ù���ijɷ��к���Na������Ҫд������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

 NaCl(l)+ K(g)����ҵ��Ӧ������Щ��ʩ����ʹ��Ӧ������Ӧ�����ƶ���___________________
NaCl(l)+ K(g)����ҵ��Ӧ������Щ��ʩ����ʹ��Ӧ������Ӧ�����ƶ���___________________ �鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com