
| A���������������ʵ������䣬������������������ | B�������������������䣬������������������ | C�������������������䣬����ˮ���������� | D���������������ʵ������䣬����CO2���������� |
| y |
| 4 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�������ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��֪2NO2(g)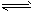 N2O4(g)
��H= -52.7kJ��mol-1��ij����С��Ϊ��̽���¶Ⱥ�ѹǿ�Ի�ѧƽ���Ӱ�죬������������ʵ�飺
N2O4(g)
��H= -52.7kJ��mol-1��ij����С��Ϊ��̽���¶Ⱥ�ѹǿ�Ի�ѧƽ���Ӱ�죬������������ʵ�飺
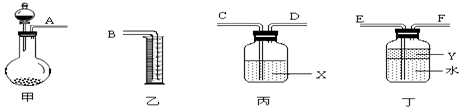
��.��С���ͬѧȡ��������ƿA��B���ֱ������ͬŨ�ȵ�NO2��N2O4�Ļ�����壬�м��ü��Ӽн��� ����A��B���뵽��ʢ��ˮ�������ձ��У���ͼ��ʾ����Ȼ��ֱ��������ձ��м���Ũ�����NH4NO3���塣��ش������й�����
��1���۲쵽��ʵ������Ϊ��_____________________________
��2����ʵ�������֪�������¶ȣ��û�ѧƽ����___________����������桱����Ӧ�����ƶ�����Ӧ��NO2��ת���ʽ�_______________�����������С�����䡱����
��.����֧�ݻ���Ϊ30mL��Ͳ�зֱ����10mLNO2���壬����Ͳǰ�˷�ա�ʵ������е�һ֧��Ͳ�����κβ���������Ϊʵ������۲�ʱ�IJ��ն���
��3��ijͬѧ���ڶ�֧��Ͳ����Ѹ������5mL������ʱ�������ɫ���һ��ʱ���������ɫ�ֱ�dz�ˡ��Խ���һ��ʱ���������ɫ�ֱ�dz��ԭ��________________________��
��4��ijͬѧ������֧��Ͳ����Ѹ������20mL����
�ٸ�ͬѧ�۲쵽�������ǣ�__________________________
���ڴ˹����У��÷�Ӧ�Ļ�ѧƽ�ⳣ����______________(���������С�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������һƿNa2SO3���壬���ܺ���NaCl��Na2SO4��BaCl2��K2CO3��K2SO4�е�һ�ֻ������ʣ�ͨ������ʵ��ȷ������Ʒ�ijɷּ�Na2SO3������������
��ȡ������Ʒ���Թ��У���ˮ��δ���������ɣ�
������ɫ�ܲ����۲죬��ɫ��Ӧ����ɫ��
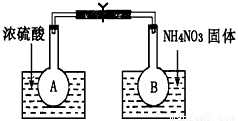
 ������ͼ��ʾ�������к�����װ���ⶨ������������(SO2�������е��ܽ���Բ���)
������ͼ��ʾ�������к�����װ���ⶨ������������(SO2�������е��ܽ���Բ���)
��ش��������⣺
�Ű����������ҷ�����ȷ������˳��Ϊ��A��___��___��____��____��B��
����������װ�ã��ɷ�Һ©����Բ����ƿ�еμ�10mLˮ����������Ͳ��___mLҺ�塣
��Һ��X��____________����������______________________________��
��Y�������Ƿ�ֹ��������������ˮ�����������Լ�����Ҫ�����______��
A.�� B.���Ȼ�̼ C.�ƾ� D.����
����ȡ��Ʒ3.00g������10.0mol/L������������(5mL)��������ɫ���壬������Ͳ��Һ������Ϊ229mL(��״��)��
�ɶԻ��ȷ�������������IJ�����_________(�����)
�ټ��װ�õ������ԣ�
����ͨ�ҡ����ĵ���ҪԤ��ע��ˮ��
�۶���ʱ��������Ͳ�ڰ�Һ����͵���ƽ��
�ָܻ������º������µ�����Ͳλ�ã�ʹ�ҡ�����Һ����ƽ��
���ڼ�װ�ý�����������һ�����ܣ�ͨ��N2��ϵͳ��SO2ȫ���ų���
�����ݳ���������Һ�м����Թ�����BaCl2��Һ�����ˡ�ϴ�ӡ�����õ���ɫ����13.98g������ʵ�������������ݣ���֪����Ʒ��Na2SO3������������_____�����е�������__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com