
�Ҵ���������Ӧ
1��ͭ˿�ھƾ���������
��ʵ��װ�á���ͼ1
��ʵ�鲽�衿
��1�� ȡһ��ͭ˿��������һ���Ƴ�����״��Ϊʲô��
��2�� ��ȼһյ�ƾ��ƣ����Ƴ�����״һ�˵�ͭ˿�Ƶ��ƾ�����
�������գ��۲�����
��3�� ������״ͭ˿���ƾ��������ƶ����۲�ʵ������
��4�� ������״ͭ˿���ƾ��������ƶ����۲�ʵ������
��5�� �ظ�ʵ�鲽��3��4�IJ�����
��������ۡ�
��1�� �ֲ�˵���ɹ۲쵽��ʵ������
��2�� �漰���Ļ�ѧ��Ӧʽ�� ��
2����֪�Ҵ����Ա�ǿ�������ظ���أ�K2Cr2O7������ɫ����������Һ������CH3COOH����ԭ����ΪCr3+����ɫ������ʵ��ԭ���������ڼ���������ʻԱ�Ƿ�ƺ�����д���÷�Ӧ�����ӷ���ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿γ�ģ������������л�ѧ����2(�ս̰�) �ս̰� ���ͣ�022
�Ҵ���������Ӧ��
(1)ȼ�գ�________��
(2)������(��ͭ�����ȴ��������±���������)��
2CH3CH2��OH��O2![]() ________��________
________��________
���ʵ������£���ȩ���ɱ������������________��
(3)�Ҵ�Ҳ�ܱ�����KMnO4��Һ������ʹ֮��ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ��
�� ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ���������Ӧ
�� ��Ӧ������ȡ������ȡ�����
��2����������ˮԡ���ò���ͬ��
�������� ���ҵ������� ��
��3�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���п��ܻ����е����ʵ������� ��Ҫ��ȥ�����ʣ����ڻ��Һ�м��� ���˿���д��ĸ����Ȼ����ͨ�����ɳ�ȥ��
a���Ȼ�����Һ�� ����b�������� c��̼��������Һ �� ��d�����Ȼ�̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ���ij�ˮ����ѧ�߶���ѧ�ڽβ��ԣ�������ѧ�Ծ����������� ���ͣ�ʵ����
ijʵ��С��������װ�ý����Ҵ���������ʵ�顣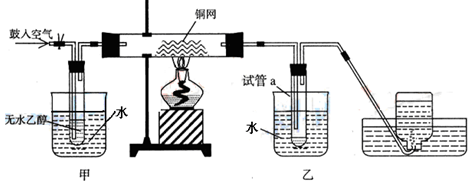
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ��
�� ��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ���������Ӧ
�� ��Ӧ������ȡ������ȡ�����
��2����������ˮԡ���ò���ͬ��
�������� ���ҵ������� ��
��3�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���п��ܻ����е����ʵ������� ��Ҫ��ȥ�����ʣ����ڻ��Һ�м��� ���˿���д��ĸ����Ȼ����ͨ�����ɳ�ȥ��
a���Ȼ�����Һ�� ����b�������� c��̼��������Һ �� ��d�����Ȼ�̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��5 3.1�� ����ϰ���������棩 ���ͣ������
ijʵ��С��������װ�ý����Ҵ���������ʵ�飮
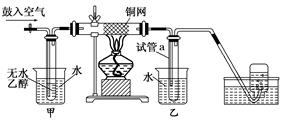
(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________.
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ���������Ӧ��________��Ӧ��
(2)��������ˮԡ���ò���ͬ��
��������________���ҵ�������________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
(4)���Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���________(��д��ĸ)��
a���Ȼ�����Һ b����
c��̼��������Һ d�����Ȼ�̼
Ȼ����ͨ��________(��ʵ���������)���ɳ�ȥ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com