
 ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʡ���ش��������⡣
ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ�飬�������о������仯����IJ������ʡ���ش��������⡣
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________��
��2����ͬѧ��ȷ����Ӧ��Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽����
�ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB��
 ��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ��
��ȡ������ҺB�μ�KSCN��Һ������Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ������Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ��� ��
��3����ͬѧ������ʵ�鷽��������ʵ�飬�����Һδ���ɫ��ԭ����
�������ӷ���ʽ��ʾ����
��4����ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ��������ͼ��ʾ�IJ������ɹ۲쵽���ɰ�ɫ������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��������д��������������صķ�Ӧ�Ļ�ѧ����ʽ ��
��5��һ��ʱ���ͬѧ���֣�3����δ������Һ��ɺ�ɫ��˵��Fe2+ ���� �ԡ��ɴ˿�֪��ʵ�����к�Fe2+������Һ���������Ƶ�ԭ���� ��
�������ƺ�Fe2+������ҺʱӦ�������� ��
 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ˮ�Ҵ��������Ʊ����������Ļ�ѧ����ʽ���£�
CH3COOH��C2H5OHCH3COOC2H5��H2O
��1���÷�Ӧ��ƽ�ⳣ������ʽK������ ����
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ�� ��� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | ����̼������Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 | |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 | |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
��ʵ��D��Ŀ������ʵ��C����գ�֤��H����������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
�ۼ���������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ִ����ѧ�߶��ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ˮ�Ҵ��������Ʊ����������Ļ�ѧ����ʽ���£�
CH3COOH��C2H5OH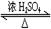 CH3COOC2H5��H2O
CH3COOC2H5��H2O
��1���÷�Ӧ��ƽ�ⳣ������ʽK������ ����
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ�� ��� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | ����̼������Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 | |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 | |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ��̨�и�����һ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��
��1��д��ʵ�����Ʊ����������Ļ�ѧ����ʽ ��
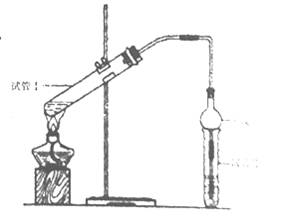
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ�II�ٲ��л���ĺ�ȣ�ʵ���¼���£�
�ĺ�ȣ�ʵ���¼����
|
ʵ���� |
�Թܢ��е��Լ� |
�Թܢ��е��Լ� |
����л���ĺ��/cm |
|
A |
2mL�Ҵ���2 mL���ᡢ1 mL 18mol/LŨ���� |
����̼������Һ |
5.0 |
|
B |
3 mL�Ҵ���2 mL���� |
0.1 |
|
|
C |
3 mL�Ҵ���2 mL���ᡢ6 mL 3mol/L���� |
1.2 |
|
|
D |
3 mL�Ҵ���2 mL���ᡢ���� |
1.2 |
��1������ܵ������ǣ� ���Թܢ���ʢ�ŵ�̼������Һ ����ܡ����ܡ�����Ϊ����������Һ��ԭ���� ��
��2��ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
��3������ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
��4������������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������и߶��ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ˮ�Ҵ��������Ʊ����������Ļ�ѧ����ʽ���£�
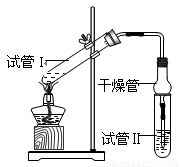
CH3COOH��C2H5OH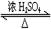 CH3COOC2H5��H2O
CH3COOC2H5��H2O
��1���÷�Ӧ��ƽ�ⳣ������ʽK������ ����
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
|
ʵ�� ��� |
�Թܢ��е��Լ� |
�Թܢ����Լ� |
����л���ĺ��/cm |
|
A |
2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� |
����̼������Һ |
5.0 |
|
B |
3mL�Ҵ���2mL���� |
0.1 |
|
|
C |
3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� |
1.2 |
|
|
D |
3mL�Ҵ���2mL���ᡢ���� |
1.2 |
��ʵ��D��Ŀ������ʵ��C����գ�֤��H����������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
�ۼ���������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com