
��10�֣����;�ˮ��������أ�K2FeO4��Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֡�
��������������������������������ͼ��ʾ��
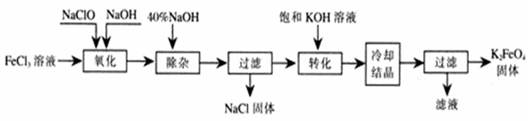
д���������������з�Ӧ�����ӷ���ʽ�� ��
��д����ת���������з�����Ӧ�Ļ�ѧ����ʽΪ ��
���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ�� �ܽ⣬Ȼ�� ��
������ⷨ������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH��
�ȵ��ʱ����������Ӧ����FeO42-���õ缫��Ӧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ӧ��Ϊ������ԭ��Ӧ����Ӧ��Ϊ���ֽⷴӦ | B����Ӧ�������������뻹ԭ�������ʵ���֮��Ϊ2��9 | C����Ӧ�ڵIJ���K2FeO4��FeΪ+6�ۣ�����ǿ�����ԣ���ɱ������ | D������2mol FeCl3������Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ6 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
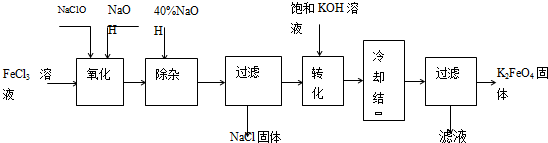
���;�ˮ��������أ�K2FeO4��Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֡�
������������������������������ͼ
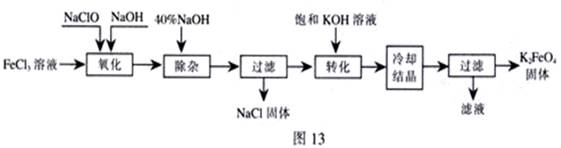
��1���������������е��������� ���ѧʽ�����Ƚ�NaClO��Na2FeO4��������ǿ�������=������ ��
�����ӡ���ȥ���� ��
��2����ת���������з����Ļ�ѧ��Ӧ����ʽΪ �������Ǽ����ķ��� ������ԭ�����ơ�
��3���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ�� �ܽ⣬Ȼ�� ��
������ⷨ��
��4������Ϊ�����������������Һ��Ȼ����������Һ�м��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡտ��һ�и�����ѧ��10�·��¿������ۣ���ѧ�� ���ͣ������
���;�ˮ��������أ�K2FeO4��Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֡�
������������������������������ͼ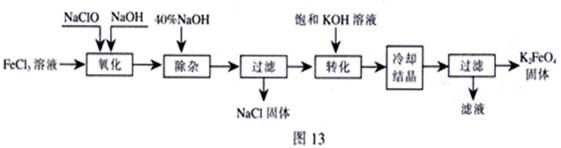
��1���������������е��������� ���ѧʽ�����Ƚ�NaClO��Na2FeO4��������ǿ�������=������ ��
�����ӡ���ȥ���� ��
��2����ת���������з����Ļ�ѧ��Ӧ����ʽΪ �������Ǽ����ķ��� ������ԭ�����ơ�
��3���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ�� �ܽ⣬Ȼ�� ��
������ⷨ��
��4������Ϊ�����������������Һ��Ȼ����������Һ�м��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com