
| HBr |
| �������� |
 �ϳ�
�ϳ�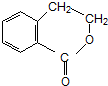 ���÷�Ӧ����ͼ��ʾ����ע����Ӧ������
���÷�Ӧ����ͼ��ʾ����ע����Ӧ������| HBr |
| �������� |
| ŨH2SO4 |
| 170�� |
| ���¸�ѹ |
| ���� |
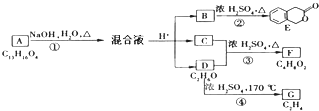
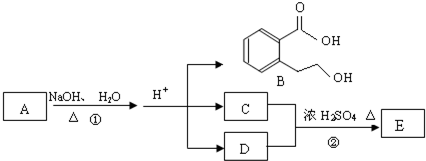
 ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ����϶�Ӧ�л���Ľṹ�������Լ���ĿҪ������⣮
����϶�Ӧ�л���Ľṹ�������Լ���ĿҪ������⣮ ��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ
��D�ķ���ʽΪC2H6O��һ�������¿�������C2H4����DΪCH3CH2OH��EΪCH2=CH2��C��CH3CH2OH��Ũ���ᡢ��������������F�����F�ķ���ʽC4H8O2��֪������F�ķ�ӦΪ������Ӧ����CΪCH3COOH��FΪCH3COOCH2CH3��A����������ˮ��Һ�����������·���ˮ�ⷴӦ���ữ�õ�B��C��D����AΪ ��
��| Ũ���� |
| �� |
| Ũ���� |
| �� |
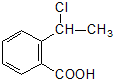
 ���еĹ�����Ϊ�Ȼ����ǻ���AΪ
���еĹ�����Ϊ�Ȼ����ǻ���AΪ ����Ӧ��Ϊ����ˮ�ⷴӦ��ȡ����Ӧ��
����Ӧ��Ϊ����ˮ�ⷴӦ��ȡ����Ӧ�� ����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ������Ȼ����ǻ����۲���FeCl3��Һ������ɫ��Ӧ��˵���������ǻ�������ܵĽṹ��ʽΪ
����Ӧ��ͬ���칹���У��ٺ����ڶ�ȡ�������ṹ������B����ͬ�����ţ������Ȼ����ǻ����۲���FeCl3��Һ������ɫ��Ӧ��˵���������ǻ�������ܵĽṹ��ʽΪ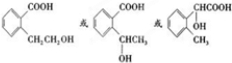 ����3�֣�
����3�֣� ��
�� �ڼ��������·�����ȥ��Ӧ����̼̼˫����Ȼ�����廯�ⷢ���ӳɷ�Ӧ���ڼ���������ˮ�����ɴ�������������Ӧ������Ŀ�����Ӧ�����̿�Ϊ
�ڼ��������·�����ȥ��Ӧ����̼̼˫����Ȼ�����廯�ⷢ���ӳɷ�Ӧ���ڼ���������ˮ�����ɴ�������������Ӧ������Ŀ�����Ӧ�����̿�Ϊ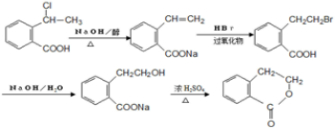 ��
�� ��
��

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
 ����CH2=CH2+H2O
����CH2=CH2+H2O| ���� |
| ���� |
 ����CH2=CH2+H2O
����CH2=CH2+H2O| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
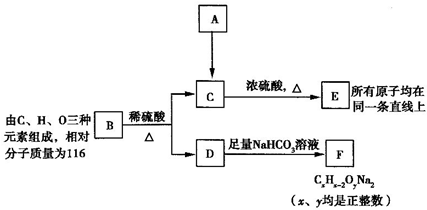
 ��ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�
��ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com