
��һ��ɫ��������������к�SO2��CO2������λͬѧ�ֱ��������������ʵ�鷽������������ɫ�������λ���ͨ������ʯ��ˮ��Ʒ����Һ�����ʯ��ˮ����ǡ�Ʒ����Һ��ɫ��֤��ԭ��ɫ���������мȺ���SO2�ֺ���CO2��
��������ɫ�������λ���ͨ����ˮ������ʯ��ˮ�������ˮ��ɫ��ʯ��ˮ����ǣ�֤��ԭ��ɫ���������мȺ���SO2�ֺ���CO2��
��������ɫ�������λ���ͨ��Ʒ����Һ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ�����Ʒ����Һ��ɫ��KMnO4��Һ��ɫ��dz��Ʒ����Һ����ɫ��ʯ��ˮ����ǣ�֤��ԭ��ɫ���������мȺ���SO2�ֺ���CO2��
��ش��������⣺
(1)��������ʵ�鷽���У���֤��ԭ��ɫ�����м���SO2���ֺ���CO2����________���������������жϵ�������________��
(2)��һλͬѧ����˼���Ժ������һ��ֻ������KMnO4��Һ�ͳ���ʯ��ˮҲ��֤����ɫ����������һ������SO2�ֺ���CO2��ʵ�鷽�������õ��˴�ҵ��Ͽɣ��������������ʵ�鷽����________��
|
�����𰸣�(1)������Ʒ����Һ��ɫ��֤����ɫ����һ������SO2��KMnO4��Һ��ɫ��dz��Ʒ����Һ����ɫ��֤��SO2�ѱ�������ȫ����ʯ��ˮ����ǣ���֤��ԭ��ɫ����Ⱥ���SO2�ֺ���CO2 ����(2)����ɫ�������λ���ͨ������KMnO4��Һ������KMnO4��Һ�ͳ����ʯ��ˮ ����������(1)CO2��SO2�ֱ��ڳ���ʯ��ˮ������KMnO4��Һ��Ʒ����Һ����ˮ����ɫ����������±���
��������������SO2��CO2����ʹ�����ʯ��ˮ����ǣ����Ը÷���ֻ��֤��ԭ���������һ������SO2������֤��һ������CO2�� ������������ˮ��ɫ��֤��ԭ���������һ����SO2����SO2����һ������ȫ���գ����Ը÷���ֻ��֤��ԭ���������һ������SO2������֤���Ƿ���CO2�� ����������Ʒ����Һ��ɫ��֤��ԭ���������һ������SO2������KMnO4��Һ��ɫ��dz��Ʒ����Һ����ɫ��֤��SO2�ѱ�������ȫ����ʯ��ˮ����ǣ���֤��ԭ��ɫ�����мȺ���SO2�ֺ���CO2�� ����(2)����KMnO4��Һ����SO2�ļ����Լ�������SO2�������Լ��������ʯ��ˮ��CO2�ļ����Լ�������ɫ�������λ���ͨ������KMnO4��Һ������KMnO4��Һ�������ʯ��ˮ����1������KMnO4��Һ��ɫ����ɫ��dz����2������KMnO4��Һ����ɫ�������ʯ��ˮ����ǣ���֤����ɫ����Ⱥ���SO2�ֺ���CO2�� |
|
(��ѧ˼ά)�öԱȷ�����������ʵĹ��Լ����죮����ʵ�鷽�������Ե����ۣ��ڽ���ʵ�鷽���Ŀ���������ʱ��ѡ���������������ʵ����ʣ��Ա��乲�ԣ�Ѱ������죬�Ӷ������жϣ�֤����������м���SO2����CO2��ʵ�����һ��ӦΪ������SO2���ڡ���SO2��֤��SO2�����ڡ�֤��CO2���ڣ� |


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+��Ba2+��Ag+��Mg2+��NH4+��Na+ |
| ������ | SO42-��SO32-��CO32-��AlO2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ������ʮһ�и�����ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��6�֣�����һ��ɫ�Ļ�����ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����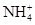 ��Cl��
��Cl��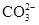 ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ�����и�����ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
��6�֣�����һ��ɫ�Ļ�����ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����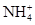 ��Cl��
��Cl��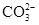 ��
��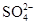 ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com