
��1)ˮ�������в���ȱ�ٵ����ʣ�A�Ƕ�������ɫ��̬���ʣ�B�Ƕ�����Ԫ����ɵĹ�̬��ɫ�����A��B������ˮ����������ԭ��Ӧ����Ӧ��ˮ�Ȳ���������Ҳ������ԭ������д��A��B�ֱ���ˮ��Ӧ�Ļ�ѧ����ʽ��_________________,______________
(2)NaAlO2��ˮ��Һ�ʼ��ԣ���ʼ��Ե�ԭ���ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ��_____________
(3)NaNH2���ɽ�������Һ����Ӧ�õ�����ˮ�������ּд��NaNH2��ˮ��Ӧ�Ļ�ѧ����ʽ__________
(4) ��֪������AgCl��AgI���ܶȻ��ֱ���1.0��10-10��1.5��10-16������200 mL����AgCl��Һ�м���������KI��Һ��Ϊʹ������Һ�в���AgI����������������KI��Һ�����ʵ���Ũ����С��______________��
(1) Cl2 + H2O  HCl + HClO 2Na2O2+
2H2O=" 4NaOH+" O2��
HCl + HClO 2Na2O2+
2H2O=" 4NaOH+" O2��
��2��AlO2���� H2O�������H+��ϣ�ʹ��Һ��c(OH��)>c(H+) ,��ʹ��Һ�ʼ��ԣ�
AlO2��+
2H2O  Al(OH)3 + OH��
Al(OH)3 + OH��
��3��NaNH2 + 2H2O="NaOH" + NH3��H2O
��4��6.0��10��11mol/L
��������
�����������1������������ɫ���嵥���������ͷ�����������ˮ��Ӧ��������ʹ����ᡢ������ˮ����������������������Ԫ����ɵĹ�����ɫ����������ˮ��Ӧʱˮ�Ȳ���������Ҳ���ǻ�ԭ��ֻ���ǹ������ơ���2��AlO2-ˮ������Al(OH)3��OH-��ԭ����AlO2-��ˮ���������H+��ϣ�c(H+)��С��c(OH-)>c(H+)����Һ�ʼ��ԡ���3��NaNH2��ˮ�������ּNaOH��NH3��H2O����4�������Ȼ�����Һ��c(Ag+)=10-5mol/L��Ϊʹ������Һ�в����⻯����������KI��ҺŨ��Ϊc����10-5/2������c/2��>1.5��10-16��c>6.0��10��11mol/L��
���㣺Ԫ�����ڱ� ������ԭ��Ӧ �����ˮ�� ��ѧ����ʽ����д �ܶȻ�����
������������Һ����γɳ�����������Q>Ksp��Ҫע��������Һ�������Ϻ������Ũ��Ϊ���ǰ��һ�롣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
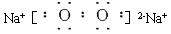
(1)��ҵ�ϣ��ڵ��A��Һ���豸�н�����������������_____������Ŀ����____________��
(2)Z![]() L��Ӧ��������__________��K�ĵ���ʽΪ__________��
L��Ӧ��������__________��K�ĵ���ʽΪ__________��
(3)д��B![]() F�����ӷ���ʽ��____________________________��
F�����ӷ���ʽ��____________________________��
(4)д��K��CO2��Ӧ�Ļ�ѧ����ʽ��_________________________��
(5)Y��NaClO��B�����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�������д���йط�Ӧ�����ӷ���ʽ��___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��������˫��ѧУ���������ģ�⻯ѧ�Ծ����������� ���ͣ������
��1)ˮ�������в���ȱ�ٵ����ʣ�A�Ƕ�������ɫ��̬���ʣ�B�Ƕ�����Ԫ����ɵĹ�̬��ɫ�����A��B������ˮ����������ԭ��Ӧ����Ӧ��ˮ�Ȳ���������Ҳ������ԭ������д��A��B�ֱ���ˮ��Ӧ�Ļ�ѧ����ʽ��_________________,______________
(2)NaAlO2��ˮ��Һ�ʼ��ԣ���ʼ��Ե�ԭ���ǣ��ñ�Ҫ�����ֺ����ӷ���ʽ��ʾ��_____________
(3)NaNH2���ɽ�������Һ����Ӧ�õ�����ˮ�������ּд��NaNH2��ˮ��Ӧ�Ļ�ѧ����ʽ__________
(4) ��֪������AgCl��AgI���ܶȻ��ֱ���1.0��10-10��1.5��10-16������200 mL����AgCl��Һ�м���������KI��Һ��Ϊʹ������Һ�в���AgI����������������KI��Һ�����ʵ���Ũ����С��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�꽨�����и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
��12�֣���ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת�� ��ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺

(1) ��ҵ�ϣ��ڵ��A��Һ���豸�н����������������� ������
(2) Z��L��Ӧ�������� ��K�ĵ���ʽΪ ��
(3) д��B��F�����ӷ���ʽ ��
(4) д��K��CO2��Ӧ�Ļ�ѧ����ʽ ��
(5) Y��NaClO��B�����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�������д���йط�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com