
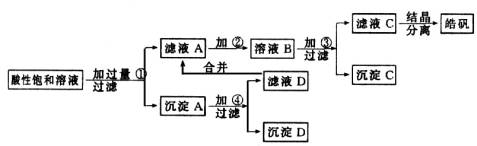
вбжЊПЊЪМЩњГЩЧтбѕЛЏЮяГСЕэЕНГСЕэЭъШЋЕФpHЗЖЮЇЗжБ№ЮЊЃКFe(OH)3ЮЊ2.7ЁЋ3.7ЃЛFe(OH)2ЮЊ7.6ЁЋ9.6ЃЛZn(OH)2ЮЊ5.7ЁЋ8.0ЁЃЪдЛиД№ЯТСагаЙиЮЪЬтЃК
(1)МгШыЕФЪдМСЂйгІЪЧ________ЃЛжївЊЗДгІЕФРызгЗНГЬЪНга________ЁЃ
(2)МгШыЕФЪдМСЂкЃЌЙЉбЁдёЪЙгУЕФгаЃКТШЫЎЁЂNaClOШмвКЁЂ20ЃЅЕФH2O2ЁЂХЈH2SO4ЁЂХЈHNO3ЕШгІбЁгУ________ЃЌЦфРэгЩЪЧ________ЁЃ
(3)МгШыЪдМСЂлЕФФПЕФЪЧ________ЁЃ
| (1)ZnЃЌZn+Cu2+
|
| зюПЊЪМЕФЫсадШмвКжагаZn2+ЁЂCu2+ЁЂFe3+КЭFe2+ЭЌЪБДцдкЃЌПЩЪзЯШМгШыZnЕЅжЪЃЌжУЛЛГіCuЃЌЭЌЪБвВЛсНЋШ§МлЬњЛЙдЮЊЖўМлЬњЃЌЗДгІМгШыЙ§СПЕФаППЩгУЯЁЫсШмвКШмвКЛиЪеЁЃШЛКѓМгШыбѕЛЏМСНЋЖўМлЬњбѕЛЏЮЊШ§МлЃЌШ§МлЭъШЋГСЕэЕФpHЮЊ3.7дкДЫpHжЕZn2+ЛЙЮДПЊЪМГСЕэЃЌЖўМлЭъШЋГСЕэЕФpHЮЊ9.6ЃЌдкДЫpHжЕZn2+вВвбОЭъШЋГСЕэЃЌЙЪЭЈЙ§ЕїНкpHжЕГ§ШЅFe2ЃЋЕФЗНЗЈЪЧааВЛЭЈЕФЁЃДЫЪБЃЌШмвКГЪМюадЃЌШчвЊЕїНкКЯЪЪЕФpHжЕЃЌШЛКѓЪЙZnSO4НсОЇГіРДЁЃ
|


 ХргХПкЫуЬтПЈЯЕСаД№АИ
ХргХПкЫуЬтПЈЯЕСаД№АИ ПЊаФПкЫуЬтПЈЯЕСаД№АИ
ПЊаФПкЫуЬтПЈЯЕСаД№АИ ПкЫуЬтПЈКгББЩйФъЖљЭЏГіАцЩчЯЕСаД№АИ
ПкЫуЬтПЈКгББЩйФъЖљЭЏГіАцЩчЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК022
ФГаЁаЭЛЏЙЄГЇЩњВњ№ЉЗЏ(ZnSO4ЁЄ7H2O)НсОЇЧАЕФЫсадБЅКЭШмвКжаКЌгаЩйСПЕФCu2+ЁЂFe3+КЭFe2+ЕШдгжЪРызгЁЃЮЊСЫГ§ШЅдгжЪЃЌЛёЕУДПОЛЕФ№ЉЗЏОЇЬхЃЌИУЙЄГЇЩшМЦШчЯТЙЄвеСїГЬЃК
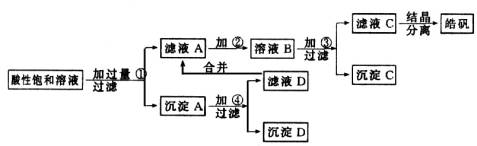
вбжЊПЊЪМЩњГЩЧтбѕЛЏЮяГСЕэЕНГСЕэЭъШЋЕФpHЗЖЮЇЗжБ№ЮЊЃКFe(OH)3ЮЊ2.7ЁЋ3.7ЃЛFe(OH)2ЮЊ7.6ЁЋ9.6ЃЛZn(OH)2ЮЊ5.7ЁЋ8.0ЁЃЪдЛиД№ЯТСагаЙиЮЪЬтЃК
(1)МгШыЕФЪдМСЂйгІЪЧ________ЃЛжївЊЗДгІЕФРызгЗНГЬЪНга________ЁЃ
(2)МгШыЕФЪдМСЂкЃЌЙЉбЁдёЪЙгУЕФгаЃКТШЫЎЁЂNaClOШмвКЁЂ20ЃЅЕФH2O2ЁЂХЈH2SO4ЁЂХЈHNO3ЕШгІбЁгУ________ЃЌЦфРэгЩЪЧ________ЁЃ
(3)МгШыЪдМСЂлЕФФПЕФЪЧ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК022
ФГаЁаЭЛЏЙЄГЇЩњВњ№ЉЗЏ(ZnSO4ЁЄ7H2O)НсОЇЧАЕФЫсадБЅКЭШмвКжаКЌгаЩйСПЕФCu2+ЁЂFe3+КЭFe2+ЕШдгжЪРызгЁЃЮЊСЫГ§ШЅдгжЪЃЌЛёЕУДПОЛЕФ№ЉЗЏОЇЬхЃЌИУЙЄГЇЩшМЦШчЯТЙЄвеСїГЬЃК
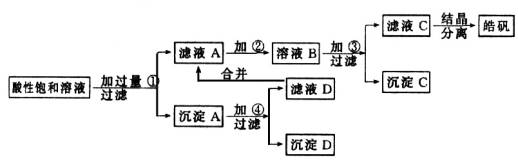
вбжЊПЊЪМЩњГЩЧтбѕЛЏЮяГСЕэЕНГСЕэЭъШЋЕФpHЗЖЮЇЗжБ№ЮЊЃКFe(OH)3ЮЊ2.7ЁЋ3.7ЃЛFe(OH)2ЮЊ7.6ЁЋ9.6ЃЛZn(OH)2ЮЊ5.7ЁЋ8.0ЁЃЪдЛиД№ЯТСагаЙиЮЪЬтЃК
(1)МгШыЕФЪдМСЂйгІЪЧ________ЃЛжївЊЗДгІЕФРызгЗНГЬЪНга__ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ _____ЁЁЁЁ _ЁЁЁЁЁЁЁЁ ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ЁЃ
(2)МгШыЕФЪдМСЂкЃЌЙЉбЁдёЪЙгУЕФгаЃКТШЫЎЁЂNaClOШмвКЁЂ20ЃЅЕФH2O2ЁЂХЈH2SO4ЁЂХЈHNO3ЕШгІбЁгУ________ЃЌЦфРэгЩЪЧ____ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ____ЁЃ
(3)МгШыЪдМСЂлЕФФПЕФЪЧ______ЁЁЁЁЁЁЁЁ __ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКЮяРэНЬбаЪв ЬтаЭЃК022

вбжЊПЊЪМЩњГЩЧтбѕЛЏЮяГСЕэЕНГСЕэЭъШЋЕФpHЗЖЮЇЗжБ№ЮЊЃКFe(OH)3ЮЊ2.7ЁЋ3.7ЃЛFe(OH)2ЮЊ7.6ЁЋ9.6ЃЛZn(OH)2ЮЊ5.7ЁЋ8.0ЁЃЪдЛиД№ЯТСагаЙиЮЪЬтЃК
(1)МгШыЕФЪдМСЂйгІЪЧ________ЃЛжївЊЗДгІЕФРызгЗНГЬЪНга__ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ _____ЁЁЁЁ _ЁЁЁЁЁЁЁЁ ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ЁЃ
(2)МгШыЕФЪдМСЂкЃЌЙЉбЁдёЪЙгУЕФгаЃКТШЫЎЁЂNaClOШмвКЁЂ20ЃЅЕФH2O2ЁЂХЈH2SO4ЁЂХЈHNO3ЕШгІбЁгУ________ЃЌЦфРэгЩЪЧ____ЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ____ЁЃ
(3)МгШыЪдМСЂлЕФФПЕФЪЧ______ЁЁЁЁЁЁЁЁ __ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com