
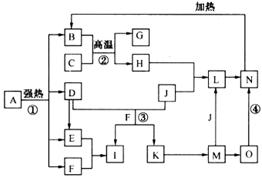
��ϸ�Ķ���ͼ����֪B��D��E��F��G�������F��K���⻯�C��H���ճ���������Ϊ�����Ľ������ʣ�J����̬�ǽ������ʣ�O�ǰ�ɫ��������B��H��L��M��N��O�к���ͬ��Ԫ��(ͼ�в��ַ�Ӧ����������ȥ)��
�밴Ҫ��ش�
(1)��Ӧ�ټ��ǷֽⷴӦ������������ԭ��Ӧ������B��D��E��F�����ʵ���֮��1��1��1��14��Aǿ�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ ��
(2)д����Ӧ���ڹ�ҵ�����ϵ�һ����;�� ��
(3)��Ӧ�۵����ӷ���ʽΪ ��
��Ӧ�ܵĻ�ѧ����ʽΪ ��
(4)����C��H�Ƴ�����ʢװ������I��Ũ��Һ����ԭ����
��
(1)2FeSO4��7H2O![]() Fe2O3+SO2��+SO3��+14H2O
Fe2O3+SO2��+SO3��+14H2O
(2)Ұ�⺸�Ӹֹ�
(3)Cl2+SO2+2H2O==4H++SO![]() +2Cl
+2Cl![]() 4Fe(OH)2+O2+2H2O==4Fe(OH)3
4Fe(OH)2+O2+2H2O==4Fe(OH)3
(4)���Ũ�������������������Ӵ�ʱ���ڱ�������һ�����ܵ�����Ĥ����ֹ�����������ᷴӦ��ʹ�������ۻ��������Կ�������������������Ũ����
������(1)2FeSO4��7H2O![]() Fe2O3+SO2��+SO3��+14H2O
Fe2O3+SO2��+SO3��+14H2O
(2)Ұ�⺸�Ӹֹ�
(3)Cl2+SO2+2H2O==4H++SO![]() +2Cl
+2Cl![]() 4Fe(OH)2+O2+2H2O==4Fe(OH)3
4Fe(OH)2+O2+2H2O==4Fe(OH)3
(4)���Ũ�������������������Ӵ�ʱ���ڱ�������һ�����ܵ�����Ĥ����ֹ�����������ᷴӦ��ʹ�������ۻ��������Կ�������������������Ũ����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
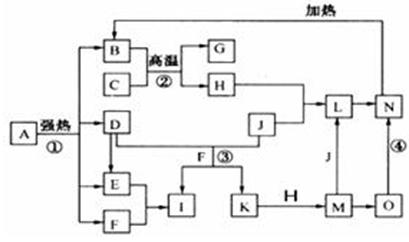
��ϸ�Ķ���ͼ����֪B��D��E��F��G�����������D���γ��������Ҫ��Ⱦ�F��K���⻯���F�ڳ�����ΪҺ̬��C��H���ճ���������Ϊ�����Ľ������ʣ�J����̬�ǽ������ʣ�O�ǰ�ɫ��������B��H��L��M��N��O�к���ͬ��Ԫ��(ͼ�в��ַ�Ӧ����������ȥ)���밴Ҫ��ش�
(1)��Ӧ�ټ��ǷֽⷴӦ������������ԭ��Ӧ������B��D��E��F�����ʵ���֮��1��1��1��14��Aǿ�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ �� ��
(2)д����Ӧ���ڹ�ҵ�����ϵ�һ����;�� �� ��
(3)��Ӧ�۵����ӷ���ʽΪ �� ��
��Ӧ�ܵĻ�ѧ����ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ϸ�Ķ���ͼ����֪B��D��E��F��G�����������D���γ��������Ҫ��Ⱦ�F��K���⻯���F�ڳ�����ΪҺ̬��C��H���ճ���������Ϊ�����Ľ������ʣ�J����̬�ǽ������ʣ�O�ǰ�ɫ��������B��H��L��M��N��O�к���ͬ��Ԫ��(ͼ�в��ַ�Ӧ����������ȥ)���밴Ҫ��ش�
(1)��Ӧ�ټ��ǷֽⷴӦ������������ԭ��Ӧ������B��D��E��F�����ʵ���֮��1��1��1��14��Aǿ�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ �� ��
(2)д����Ӧ���ڹ�ҵ�����ϵ�һ����;�� �� ��
(3)��Ӧ�۵����ӷ���ʽΪ �� ��
��Ӧ�ܵĻ�ѧ����ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ���ݶ��и���12���¿���ѧ�Ծ� ���ͣ�ʵ����
��ϸ�Ķ���ͼ����֪B��D��E��F��G�����������D���γ��������Ҫ��Ⱦ�F��K���⻯���F�ڳ�����ΪҺ̬��C��H���ճ���������Ϊ�����Ľ������ʣ�J����̬�ǽ������ʣ�O�ǰ�ɫ��������B��H��L��M��N��O�к���ͬ��Ԫ��(ͼ�в��ַ�Ӧ����������ȥ)���밴Ҫ��ش�
(1)��Ӧ�ټ��ǷֽⷴӦ������������ԭ��Ӧ������B��D��E��F�����ʵ���֮��1��1��1��14��Aǿ�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ �� ��
(2)д����Ӧ���ڹ�ҵ�����ϵ�һ����;�� �� ��
(3)��Ӧ�۵����ӷ���ʽΪ  �� ��
�� ��
��Ӧ�ܵĻ�ѧ����ʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ����12���¿���ѧ�Ծ� ���ͣ�ʵ����
��ϸ�Ķ���ͼ����֪B��D��E��F��G�����������D���γ��������Ҫ��Ⱦ�F��K���⻯���F�ڳ�����ΪҺ̬��C��H���ճ���������Ϊ�����Ľ������ʣ�J����̬�ǽ������ʣ�O�ǰ�ɫ��������B��H��L��M��N��O�к���ͬ��Ԫ��(ͼ�в��ַ�Ӧ����������ȥ)���밴Ҫ��ش�
(1)��Ӧ�ټ��ǷֽⷴӦ������������ԭ��Ӧ������B��D��E��F�����ʵ���֮��1��1��1��14��Aǿ�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ �� ��
(2)д����Ӧ���ڹ�ҵ�����ϵ�һ����;�� �� ��
(3)��Ӧ�۵����ӷ���ʽΪ �� ��
��Ӧ�ܵĻ�ѧ����ʽΪ �� ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com