
���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�á����������Ʊ��ߴ��ȵ����ᣨ����ṹʽΪ �������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ�
�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȡ�
���������գ�
��1����Ԫ��ԭ�Ӻ������������Ų�ʽΪ ��NaH2PO2���漰������Ԫ�أ����ǵ�ԭ�Ӱ뾶��С�����˳��Ϊ ��
��2����ԭ�Ӻ����� �ֲ�ͬ�����ĵ��ӡ�
��3��д������ͬ���ڵ�����Ԫ���У����Ӱ뾶��С��Ԫ�أ�������������Ӧˮ����ĵ��뷽��ʽ ��
��4�������������Ҫ�����Ƹ������¯ˮ����ʳƷ��ҵ������ҵ�������������ˮ��Һ�����Ե�ԭ����_______________________________________________________��
��5�������������Ϊ�����������֮����ȥ����ˮ���ӵIJ����ṹʽΪ �����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ____________��
��6���������ƣ�NaH2PO2�������ڻ�ѧ��������ѧ��������Һ�к���Ni2+��H2PO2������һ���������ܷ������·�Ӧ��__Ni2++__H2PO2��+ ��__Ni + ___H2PO3��+ ������ɲ���ƽ������Ӧ���ӷ���ʽ���÷�Ӧ�Ļ�ԭ������__________��
��1��3S23P3 �� H<O<P<Na
��2��3
��3��H+ + AlO2- + H2O
 Al(OH)3
Al(OH)3
 Al3+
+ 3OH- ��2�֣�
Al3+
+ 3OH- ��2�֣�
��4�������������ӵ���̶ȴ���ˮ��̶� ��2�֣�
��5��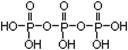 �� Na5P3O10
�� Na5P3O10
��6��Ni2++H2PO2��+H2O�� Ni+H2PO3��+2H+ ��2�֣� Ni
��������
�����������1��Hԭ����1�����Ӳ㣬O��2�����Ӳ㣬P��Na��3�����Ӳ���Na�������ٰ뾶�ʰ뾶��С˳��ΪH<O<P<Na��
��2����ԭ�ӵ����Ų�ʽΪ1S22S22P4��ͬ�������������ͬ����3�ֲ�ͬ�����ĵ��ӣ�
��3��H2PO2��Ϊ��������ӣ�����Һ�м���ˮ�����ܵ��룬ˮ��Һ�����Ե�ԭ��˵�������̶ȴ���ˮ��̶ȣ��Ե���Ϊ����
��4��������Ӽ���ˮӦ�������ǻ�����ˮ��Ӧ�����������ƣ��׳ơ����ơ������������������ǻ��ⱻ��ȡ���IJ��
��6������������ԭ��Ӧ�����غ���ƽ��������Ni2+���ɻ�ԭ����Ni��
���㣺���黯ѧ���������ʽṹ��������ԭ��Ӧ���й����⡣


 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
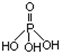 ���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹʽ��ͼ�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȣ�
���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹʽ��ͼ�������������ƣ��׳ơ����ơ����dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
(1)HgS��O2�ķ�Ӧ�У���������____________��ÿ����1 mol Hgת�Ƶ��ӵ����ʵ���Ϊ____________ mol��
(2)���ij������������Һ������������a mol����������ʱ���ɻ��
(3)����ұ������Ƶȹ�ҵ�����IJ��Ϸ�չ�����蹯���仯���������Ҳ�������࣬��֮�ŷų����ĺ����ķ�ˮҲ�������أ����ѳ�Ϊ������Σ���ϴ�Ĺ�ҵ��ˮ֮һ�����´�������(Hg2+)��ˮ�ķ��������ʵ�õ���____________(�����)��
A.��Hg2+�ķ�ˮ�м���Na2S�ȿ���������
B.���������ԭ��
C.��ⷨ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����10�·��¿���ѧ�Ծ� ���ͣ������
��4�֣������ʼ��仯�����ڹ�ҵ�����Ϳ�ѧʵ�����й㷺����;�����Ŀ�Դ����ɰ��HgS������ɰ�����ķ�Ӧ֮һΪ(�ڼ��������½���)��HgS + O2 = Hg + SO2
��ش�
HgS��O2�ķ�Ӧ�У��������� ��ÿ����1molHgת�Ƶ��ӵ����ʵ���Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣������ʼ��仯�����ڹ�ҵ�����Ϳ�ѧʵ�����й㷺����;�����Ŀ�Դ����ɰ��HgS������ɰ�����ķ�Ӧ֮һΪ(�ڼ��������½���)��HgS + O2 = Hg + SO2
��ش�
HgS��O2�ķ�Ӧ�У��������� ��ÿ����1molHgת�Ƶ��ӵ����ʵ���Ϊ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com