

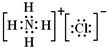
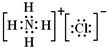
| ||
| ||
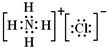 ��
��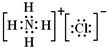 ��
��
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(5��)��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�A��B��H�����壬����֮��������ת����
ϵ��(��Ӧ�����ɵ�ˮ����ȥ)
��ش��������⣺
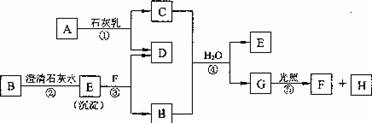 (1)E�� ��F�� (�ѧʽ)��
(1)E�� ��F�� (�ѧʽ)��
(2)C�������ճ������п��� ����
(3)д����Ӧ�ٵĻ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��C��Ϊ�������ʣ�C��ˮ��Ӧ����D����������壬D��H����ɫ��Ӧ���ʻ�ɫ����ͨ��״����E��һ�ְ�ɫ��������ˮ�����������NaOH��������ɷ������ֽⷴӦ������Ӧ���������ɵ�ˮ��������������ȥ��
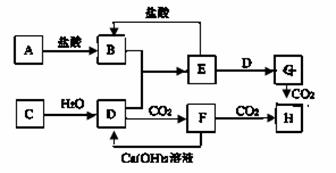
��ش��������⣺��1��B�� ��H�� �����ѧʽ��
��2��д��Dת��ΪF�����ӷ���ʽ ��
��3��E��D��Һ��Ӧ����G��д���˷�Ӧ�Ļ�ѧ����ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��ͨ�������ѧ�߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com