
��10�֣� (1)����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA��1mol NaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ�� ��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ
(2)������B����C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��
��ij�л�����ȫȼ�գ����ɱ�״����CO2�����Ϊ4.48 L��H2O������Ϊ5.4g��
�����л��������Ϊ4.6g�����л���ķ���ʽΪ
�����л��������Ϊ6.2g���Ҵ��л���lmol�ܺ������Ľ����Ʒ�Ӧ����lmolH2�����л���Ľṹ��ʽΪ (�����ǻ���������ͬһ��̼ԭ����)
(1) CH2��C(CH3)COOH��CH3CH��CHCOOH��CH2��CHCH2COOH
(2)CH3��CH2��CH2��OH��(3)��C2H6O ��HO��CH2��CH2��OH
����������1��A����ʹBr2�����Ȼ�̼��Һ��ɫ��˵������̼̼˫����1molA��1mol NaHCO3����ȫ��Ӧ�����Ժ���1���Ȼ�������A�Ľṹ��ʽΪCH2��C(CH3)COOH. ��A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽΪCH3CH��CHCOOH��CH2��CHCH2COOH��
��2��������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%�����е�̼ԭ������60��60%��12��3�����е���ԭ������60��13.33%��1��8�������ԭ�ӵĸ����ǣ�60��36��8����16��1�����Է���ʽΪC3H8O��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����˵��B��1���������ṹ��ʽΪCH3��CH2��CH2��OH��
��3����״����CO2�����Ϊ4.48 L�����ʵ�����0.2mol��H2O������Ϊ5.4g�����ʵ�����0.3mol��
������л��������Ϊ4.6g������������غ㶨�ɿ�֪���μӷ�Ӧ��������0.2��44��5.4��4.6��9.6g�����ʵ�����0.3mol�������л����к��е���ԭ����0.4��0.3��0.6��0.1mol�����C��H��O��ԭ�Ӹ���֮����2�U6�U1.���������ʽΪC2H6O��
�����л��������Ϊ6.2g������������غ㶨�ɿ�֪���μӷ�Ӧ��������0.2��44��5.4��6.2��8.0g�����ʵ�����0.25mol�������л����к��е���ԭ����0.4��0.3��0.5��0.2mol�����C��H��O��ԭ�Ӹ���֮����1�U3�U1.���������ʽΪC2H6O2���������ʿ�֪��Ӧ���Ƕ�Ԫ�������ṹ��ʽΪHO��CH2��CH2��OH��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��R1��R2��R3����������
��R1��R2��R3����������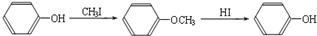









�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ˮһ��2010��2011ѧ���һ��ѧ����ĩ���Ի�ѧ�������� ���ͣ�022
(1)����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1 mol��A��1 mol��NaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ��________��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ________
(2)������B����C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60���������������Ϊ13.33����B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��________
(3)A��B��ŨH2SO4�����·���������Ӧ�������л���D�Ľṹ��ʽ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)
(1)����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA��1mol NaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ�� ��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ
(2)������B����C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��
(3)A��B��ŨH2SO4�����·���������Ӧ�������л���D�Ľṹ��ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(10��)
(1)����֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA��1mol NaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ�� ��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ
(2)������B����C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��
(3)A��B��ŨH2SO4�����·���������Ӧ�������л���D�Ľṹ��ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���л���A�����������Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������
ˮ������Ҳһ������A��������Է���������ȣ��Ҽ��ܷ���������Ӧ���ܷ���������Ӧ��
��A�Ľṹ��ʽΪ ��
��2����ѧ�ҷ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�
NO����������NO�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����
��֪M�ķ�����Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��
2.20%��18.50%����M�ķ���ʽ��_____________________��
��3������֧���Ļ�����B�ķ���ʽΪC4H6O2��B����ʹBr2�����Ȼ�̼��Һ��ɫ��1mol B��1mol NaHCO3����ȫ��Ӧ����B�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com