
 m��2cm��
m��2cm�� ������
������ ƿ��
ƿ��| A��ʹ����ֽ�����������ƹ��� | B������ƿ��ԭ��������������ˮ |
| C���ܽ��õ��ձ�δ�����ϴ�� | D���ý�ͷ�ιܼ�ˮ����ʱ���ӿ̶��� |
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ______������ţ�����ȱ�ٵ������� ��
______������ţ�����ȱ�ٵ������� ��| A����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ |
| B����������ƽȷ��ȡ�����Na2CO3?10H2O����������������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ� |
| C��������ȴ����Һ�ز�����ע��250mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
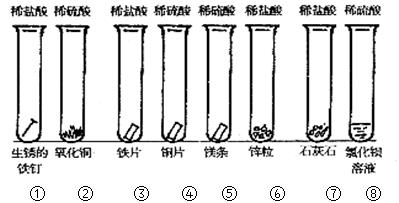
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
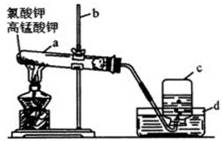
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
���ʵ���Ũ�ȵ�NaOH��Һʱ�����в�����������ҺŨ�Ȼ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һ��δϴ���ձ��Ͳ������� |
| B���ߵ�ҡ����Һ����Һ����ڿ̶��ߣ��ٲ���ˮ���̶��� |
| C������ʱ�����ӿ̶��� |
| D��ԭ����ƿ������ˮϴ����δ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �IJ��������ҳ�����ͼʾ����ȷ��ʵ����� �� ��
�IJ��������ҳ�����ͼʾ����ȷ��ʵ����� �� ��
| A����ȥCO��CO2 | B����ȡʱ���Һ | C��ϡ��Ũ���� | D�����Թ��еμ�Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������е���Ԫ��ʱ�����������NaOH��Һ��ϼ��Ⱥ���ϡ��������ữ |
| B��Ϊ��߸��������Һ�����������������Ὣ���������Һ�����ữ |
| C����ȥ���еļױ������μӸ��������Һ��NaOH��Һ��Ȼ���÷�Һ©����Һ |
| D������CH3��CH==CH��CHO������ȩ����������ˮ��������Һ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com