
���� ��1���ٸ��ݹ�ϵʽ3Fe��Fe3O4������Ʒ��Fe��������
�ڸ���FeԪ����������CԪ������������n=$\frac{m}{M}$����n��C�����ٸ���ԭ���غ����n��CO2��������V=nVm���������̼�����
��2��ȡϡ�ͺ���Һ25mL�������к͵ζ�����15.5mL���ᣬ��ʱ��Һ������ΪNaCl�������к�250mLϡ��Һ��Ҫn��HCl���������������غ�����к�250mL��Һ����Һ��n��NaCl�����ٸ����������غ����n��FeCl3�����������������ʵ���Ũ�ȣ�
��3������ȫ��Ӧ������ʣ�࣬˵��������ȫ��Ӧ������Һ������Ϊ����������n��NO��=$\frac{3.584L}{22.4L/mol}$=0.16mol������Nԭ���غ�����������������ʵ�����
�ڸ�����ԭ���غ㡢ת�Ƶ����غ������������ʵ������ٸ�������������ʽ������������������
��4�����Լ�����2molFe2+��1mol Fe 3+��������Ԫ�ص��������ٸ������������ӵ�������֮�Ⱥ����������ȣ����������Ӽ���ѧʽ��
��� �⣺��1����������к�������Ϊm g����
3Fe��Fe3O4
168 232
m g 16.24g
��168��232=mg��16.24g�����m=11.76
�ʴ�Ϊ��11.76��
��CԪ������=11.802g-11.76g=0.042g��n��C��=$\frac{0.042g}{12g/mol}$=0.0035mol������ԭ���غ�n��CO2��=0.0035mol���ʶ�����̼���=0.0035mol��22.4L/mol=0.0784L=78.4mL���ʴ�Ϊ��78.4��
��2��ȡϡ�ͺ���Һ25mL�������к͵ζ�����15.5mL���ᣬ��ʱ��Һ������ΪNaCl���к�250mLϡ��Һ��Ҫn��HCl��=0.100mol/L��0.0155L��$\frac{250}{25}$=0.0155mol��16.8g 5%���ռ���Һ��n��NaOH��=$\frac{16.8g��5%}{40g/mol}$=0.021mol�������������غ㣬250mL��Һ������ζ�����Һ��n��NaCl��=n��NaOH��=0.021mol���ٸ����������غ㣺3n��FeCl3��+0.001mol+0.0155mol=0.021mol�����n��FeCl3��=0.0015mol����ԭ��Һ��Fe3+�����ʵ���Ũ��=$\frac{0.0015mol}{0.01L}$=0.15mol/L��
��ԭ��Һ��Fe3+�����ʵ���Ũ��Ϊ0.15mol/L��
��3������ȫ��Ӧ������ʣ�࣬˵��������ȫ��Ӧ����Һ������Ϊ����������n��NO��=����ȫ��Ӧ������ʣ�࣬˵��������ȫ��Ӧ����Һ������Ϊ����������n��NO��=$\frac{3.584L}{22.4L/mol}$=0.16mol��n��HNO3��=1mol/L��0.7L=0.7mol������Nԭ���غ��n[Fe��NO3��2]=$\frac{0.7mol-0.16mol}{2}$=0.27mol����Fe��NO3��2�����ʵ���Ϊ0.27mol��
������������ʵ���Ϊxmol��������ԭ���غ�ã��μ�������ԭ��Ӧ��n��Fe��=0.27mol-xmol��
����ת�Ƶ����غ��xmol+0.16mol��3=��0.27-x��mol��2��x=0.02��ԭ����������=2.020g+��0.27-0.02��mol��56g/mol=16.020g��
�������������=$\frac{0.02mol��89g/mol}{0.02mol��89g/mol+16.020g}$��100%=10%��
��ԭ������Ƭ���������������Ϊ10%��
��4������2molFe2+��1mol Fe3+������Ԫ�ص�����Ϊ3 mol��56g/mol=168g �����������ӵ�������֮��Ϊ11��12
���������ӹ���168g��$\frac{11}{12}$=154g���Ҵ���7 mol�����
����SO42-ֻ��1mol��96g��
ʣ�����������ӹ�58g���Ҵ���5mol����ɣ�
��Ϊ5mol OH-Ϊ85g����˵�����������ֻ��Ϊ�������ҵ�ɶ��O2-����ΪOH-��O 2-�����ʵ����ֱ�Ϊx��y��
��x+2y=5��17x+16y=58
���x=2mol��y=1.5mol
������������ӵ����ʵ���֮����2��4��3
���Ի�ѧʽΪFe6��SO4��2��OH��4O3��2FeSO4•2Fe��OH��2•Fe2O3��2FeSO4•Fe��OH��2•Fe3O4•H2O����X�Ļ�ѧʽΪFe6��SO4��2��OH��4O3��2FeSO4•2Fe��OH��2•Fe2O3��2FeSO4•Fe��OH��2•Fe3O4•H2O��
���� ���⿼��������㣬��Ŀ�������ϴ��̸��ӣ�Ϊ�״���Ŀ�����ؿ���ѧ���������������������ȷ��Ӧ�����ǹؼ���ע�������غ�˼����㣬�ѶȽϴ�


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ��صĵ缫һ��Ҫ�����ֲ�ͬ�Ľ������ | |
| B�� | ԭ��صĸ����õ��ӣ�������ԭ��Ӧ | |
| C�� | ԭ��ع���ʱ�����������ϲ������Ӳ������·���� | |
| D�� | ԭ����ܽ���ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ȳ���Ƿ����H2S��ʪ��Ĵ���Ǧ��ֽ | |
| B�� | ����ζ�NaHCO3��Һ����̪ | |
| C�� | ��������Ƿ���ȫˮ�⣺��ˮ | |
| D�� | �����Ȼ����Ƿ�����ʪ���pH��ֽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������������в��ִ���ת��Ϊ���ᣬ�����γɸ���������ʹ�ƾ������������ζ�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30g/L���������������������ƣ�Ӧ������2.0g/L��
������������в��ִ���ת��Ϊ���ᣬ�����γɸ���������ʹ�ƾ������������ζ�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30g/L���������������������ƣ�Ӧ������2.0g/L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
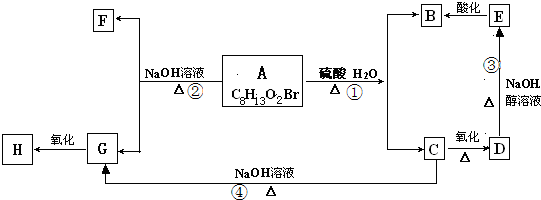
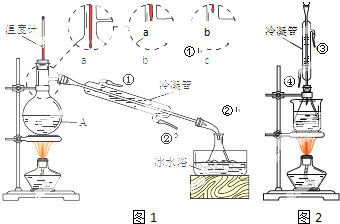
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ױ��ķ���ʽΪ��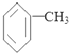 | |
| B�� | �ױ�����������ԭ�Ӷ�����ͬһƽ�� | |
| C�� | �ױ���һ��ȡ������5��ͬ���칹�壬���ǵ��۵㡢�е������ͬ | |
| D�� | �ױ��ͱ���Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com