
| ��� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 0.00 | 20.10 |
| 2 | 1.00 | 20.90 |
| 3 | 0.00 | 21.10 |
���� ��1���ٸ�����ؾ���ǿ�����ԣ�Ҫ�ữ���������Һ��Ҫѡ����ԭ�Ե��ᣬ�¶�Խ�߷�Ӧ����Խ�죬����ɫʱ��Խ�̣�
�����������£�������������������������ɶ�����̼����������ԭ���������ӣ�ͬʱ����ˮ��
��2����������Һֻ��ʢ������ʽ�ζ����У��������д����ö����·�Ӧ���ʼӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��֤���ﵽ�յ㣻
��ƽ��ֵ�������ĸ�����ص������������ı�ֵ�����ݸ�����غͲ���֮��Ĺ�ϵʽ���㣻
�ܸ���C=$\frac{n}{V}$�жϲ���������n��V��Ӱ���жϣ�
��� �⣺��1���ٸ�����ؾ���ǿ�����ԣ�Ҫ�ữ���������Һ��Ҫѡ����ԭ�Ե��ᣬһ��ѡϡ���ᣬ�¶�Խ�߷�Ӧ����Խ�죬����ɫʱ��Խ�̣�������ɫ����ʱ��tA��tB���ʴ�Ϊ�����ᣬ����
�����������£�������������������������ɶ�����̼����������ԭ���������ӣ�ͬʱ����ˮ�����������ӷ�Ӧ����ʽΪ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
�ʴ�Ϊ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
��2����������Һֻ��ʢ������ʽ�ζ����У��������Ը��������ҺӦ��ʢ������ʽ�ζ����У�����������ӱ���ԭ���ɵ��������д����ö����·�Ӧ���ʼӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��֤���ﵽ�յ㣬
�ʴ�Ϊ����ʽ����Ӧ�����ɵ������Ӿ��д����ã����������ɫ��ӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��
�۵���������������һ�κ͵ڶ������ϴ�����Ҫ��ȥ��������KMnO4��Һ��ƽ�����=$\frac{��20.10-0.00��+��20.90-1.00��}{2}$mL=20.00mL��
����Ʒ�Ĵ���Ϊx��
5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O
450g 2mol
5.0x��$\frac{1}{10}$g ��0.1��0.020��mol
x=$\frac{0.1��0.020��450}{2��5.0��0.1}$=90.00%��
�ʴ�Ϊ��90.00%��
��A����ʽ�ζ���ˮϴ��δ�ô���Һ��ϴ���ᵼ�����Ը������Ũ��ƫС����Ҫ����������ƫ�ⶨֵƫ����ȷ��
B����ƿ����ˮ����ʵ����Ӱ�죬�ʴ���
C�����ܼ��첿�������ݣ��ζ�����ʧ���ᵼ�²ⶨ����������ƫ�ⶨֵƫ����ȷ��
D����С�Ľ���������KMnO4��Һ������ƿ�⣬�ᵼ�²ⶨ����������ƫ�ⶨֵƫ����ȷ��
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ����²ⶨ����������ƫС���ⶨֵƫС���ʴ���
��ѡACD��
���� ���⿼����̽�����ʵ���ɼ��京���IJⶨ���ѶȽϴ�ע�����ʵ������������ܻ�����������IJ���������ע�����ʵ�����Ҫ���ע�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
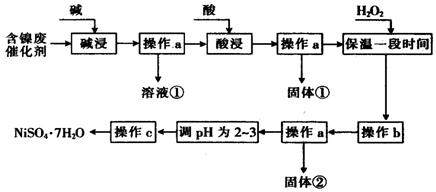
| ������ | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| Fe��OH��2 | 7.6 | 9.7 |
| Ai��OH��2 | 7.1 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ��/min | ��ƽ��ʱ��ϵ�����ı仯/kJ | ||||
| CO | H2O | CO2 | H2 | ||||
| �� | 1 | 4 | 0 | 0 | t1 | �ų�������32.8kJ | |
| �� | 2 | 8 | 0 | 0 | t2 | �ų�������Q | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ѹǿ/MPa �������/% �¶�/�� | 2.0 | 4.0 | 6.0 |
| 700 | 55.0 | a | b |
| 850 | c | 75.0 | d |
| 950 | e | f | 85.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ҫ���Ȳ��ܽ��еĻ�ѧ��Ӧһ�������ȷ�Ӧ | |
| B�� | ��ѧ��Ӧ�����仯�����������������ǹ��ܡ����ܵ� | |
| C�� | ��ѧ��Ӧ�����е������仯��Ҳ���������غ㶨�� | |
| D�� | ��Ӧ�������������������������ʱ���������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com