
(1)������ͷ�к�����?
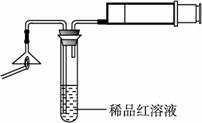
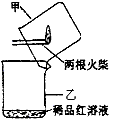
����������������ͼ��ʾʵ��װ���е�©�����棬��һ��ȼ�ŵĻ���ȼ����������ע���������������û��ȼ�ղ���������ͨ��ϡƷ����Һ���۲쵽Ʒ����Һ��ɫ��?
��ȼ�ղ�����������һ������������������?
�ڿ���������Թ���Ʒ����Һ���Լ�������������(����)��?
A.ϡ�������������Һ? B.����ʯ��ˮ? C.ϡ��ˮ? D.�ռ���Һ?
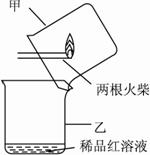
��ijͬѧ���������ͼ��ʾ��������ʵ���Ϊ��㡣���IJ����ǣ�?
i.��ͼ����ʾ���ͷȼ���꣬�����Ƴ����?
ii.����������������������������������?
(2)�ⶨ���ͷ��KClO3�ĺ���?
��Ҫʵ�鲽�����£�?
��.��ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45 g��?
��.����������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����?
��.��װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����?
��.���������Ƶ�������Ϊ1.435 g��?
��ʵ���з����ķ�Ӧ�ǣ�KClO3+3NaNO2+AgNO3��AgCl��+3NaNO3+KNO3,����NaNO2
����������������������Ӧ��AgNO3��NaNO2����Ҫ������ԭ��������������������������
��ʵ���û��ͷ��KClO3����������Ϊ����������?
������ڢ�����δϴ�Ӳ��������KClO3������������������������(�ƫ��ƫС������Ӱ�족����ͬ)������ڢ����У�δϴ��AgCl���������KClO3������������������������������
(1)��SO2(���������)?
��A��C?
��Ѹ�ٽ����ձ��������ձ��ϣ���������ձ�(����������Ҳ�ɸ���)?
(2)�ٻ�ԭ?
ȷ��KClO3����Ԫ��ȫ��ת��ΪAgCl����?
��50%?
��ƫСƫ��
������(1)����ɿ�KClO3Ϊ��������MnO2Ϊ������SΪ��ȼ���ȼ�ղ�����SO2��?
SO2���˿���Ʒ������⣬������SO2ˮ��Һ��ԭ�Խ�ǿ��������ɫ�����������棬�ʿ�ѡϡKMnO4��Һ��ϡ��ˮ��?
����ȼ�ղ���SO2�ܶȱȿ����ʿ�Ѹ�ٽ����ձ��������ձ��ϣ�����SO2�ܶ�ʹSO2�Ӵ�Ʒ�죬�������?
(2)��NaNO2��Ӧ���ΪNaNO3��NԪ�ػ��ϼ����ߣ�Ϊ��ԭ�������߹���ʹKClO3��ַ�Ӧ��
���ھ�Clԭ���غ�,1.435 gΪAgCl��n(KClO3)= n(AgCl)=0.001 mol��m(KClO3)=1.225 g��
�۲�ϴ��������KClO3�в�����MnO2�����������յ�m(KClO3)��С��?
��ϴAgCl�����������ʸ��ţ�ʹm(AgCl)��������m(KClO3)����


 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��6 3.1����ͻ����ijЩ�ɷֵļ�����ϰ���������棩 ���ͣ������
���ͷ��ͨ����������ء��������̡�������ʡ�ij�о���ѧϰС����л��ͷ���й����ʵ�ʵ��̽����

(1)ijͬѧ�о��˽̲��е�ʵ��װ�ú��������ͼ��ʾ��������ʵ���Ϊ��㡣���IJ����ǣ�
A����ͼ����ʾ���ͷȼ����ʱ�������Ƴ����
B��________________________________________________________________________
________________________________________________________________________��
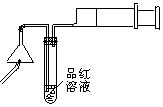
(2)�ⶨ���ͷ��KClO3�ĺ�����Ҫʵ�鲽�����£�
A����ȡ���ͷ��С�����飬�Ƶ�������Ϊ2.45 g ��
B������������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����
C����װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����
D�����������Ƶ�������Ϊ1.435 g��
��ʵ���з����ķ�Ӧ��KClO3��3NaNO2��AgNO3===AgCl����3NaNO3��KNO3����Ӧ��AgNO3��NaNO2����Ҫ������ԭ����
________________________________________________________________________��
��ʵ���û��ͷ��KClO3����������Ϊ__________��
�����B������δϴ�ӳ������������KClO3������������____________(�ƫ����ƫС������Ӱ�족����ͬ)�����C������δϴ��AgCl���������KClO3������������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��16�֣����ͷ��ͨ����������ء��������̡�������ʡ�ij�о���ѧϰС����л��ͷ���й����ʵ�ʵ��̽����
 �ż�����ͷ�к�����
�ż�����ͷ�к�����
����������������ͼ��ʾʵ��װ����©�����棬��һ��ȼ�ŵĻ���ȼ������������ע���������������û��ȼ�ղ���������ͨ��ϡƷ����Һ���۲쵽Ʒ����Һ��ɫ��
�� ȼ�ղ�����������һ������ ��
�� ����������Թ���Ʒ����Һ���Լ��� �����ţ���
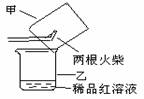 A��ϡ�������������Һ B������ʯ��ˮ C��ϡ��ˮ D���ռ���Һ
A��ϡ�������������Һ B������ʯ��ˮ C��ϡ��ˮ D���ռ���Һ
�� ijͬѧ�������ͼ��ʾ��������ʵ���Ϊ��㡣���IJ����ǣ�
������ͼ����ʾ���ͷȼ����ʱ�������Ƴ����
���� ��
�Ʋⶨ���ͷ��KClO3�ĺ���
��Ҫʵ�鲽�����£�
�� ��ȡ���ͷ��С�����飬�Ƶ�����Ϊ2.45g��
�� ����������ˮ��ֽ��ݺ���ˡ�ϴ�Ӳ�����
�� ��װ����Һ��ϴ��Һ���ձ��м��������NaNO2��Һ��AgNO3��Һ��ϡ���ᣬ���裬��ַ�Ӧ���ˡ�ϴ�ӳ�����
�� ���������Ƶ�������Ϊ1.435g��
�� ʵ���з����ķ�Ӧ�� KClO3 + 3NaNO2 + AgNO3 = AgCl��+ 3NaNO3 + KNO3������NaNO2�� ������Ӧ��AgNO3��NaNO2����Ҫ������ԭ����
�� ʵ���û��ͷ��KClO3����������Ϊ ��
�� ����ڢ�����δϴ�ӳ������������KClO3������������ ���ƫ����ƫС��������Ӱ�족����ͬ��������ڢ�����δϴ��AgCl���������KClO3������������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com