
Ϊ̽��Fe(NO3)2���������ȷֽ����Ͳ�������ʣ�ij��ѧС�鿪չ����̽����
���������ϡ�2KNO3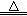 2KNO2+O2�� Fe(NO3)2
2KNO2+O2�� Fe(NO3)2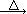 FexOy+NO2��+O2��
FexOy+NO2��+O2��
ʵ��һ��̽��Fe(NO3)2�ȷֽ�����������Ԫ�صļ�̬����С���ͬѧ���ֽ��Ĺ����������������ϡH2SO4�õ���Ӧ������Һ����������̽��ʵ�顣
��1�����ᴿ���롿
����һ����Ԫ��ֻ��+2�ۣ�
���������Ԫ�� ��
����������Ԫ�ؼ���+2������+3�ۡ�
��ʵ�����������һ����Һ�е���KSCN��Һ������һ����Һ�е�������KMnO4ϡ��Һ��
��2����ʵ������ʵ��� ��ʵ��� ��
��3����ʵ����ۡ��������������Fe(NO3)2�ֽ�Ļ�ѧ����ʽ�� ��
ʵ�����
��4��̽��Fe(NO3)2�ȷֽ������������ʡ�С����ͬѧ����������ʵ�飬�����ʵ���ȱ�������ݡ���ѡ�Լ�����Ʒ��ŨH2SO4��Һ��4mol/LNaOH��Һ��0.1mol/LBaCl2��Һ�������ǵ�ľ����0.1mol/L����KMnO4��Һ������ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����Fe(NO3)2�������Թ��У����ȷֽ⡣ | ��˵���ֽ�����������к���NO2�� |
| ����2������������������ͨ��ʢ������ ��Ũ�����ϴ��ƿ�� �����һ�����ڼ��顣 | ��˵���ֽ�����������к�O2�� |
��17�֣���1��ֻ��+3�ۣ�1�֣�
��2����Һ����Ѫ��ɫ��2�֣�������������Ҳ���֣� ��Һ�Ϻ�ɫ����ɫ��2�֣�
��3��4Fe(NO3)2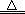 2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣�
2Fe2O3+8NO2��+O2����2�֣���ѧʽ�������֣�ϵ������1�֣���д������1�֣�
��4��ʵ�鲽�� Ԥ������ͽ��� ����1��ȡ����Fe(NO3)2�������Թ��У����ȷֽ⡣ �к���ɫ���������2�֣�������������Ҳ���֣���˵���ֽ�����������к���NO2�� ����2������������������ͨ��ʢ������ 4mol/LNaOH��Һ��2�֣���Ũ�����ϴ��ƿ���ô����ǵ�ľ����2�֣������һ�����ڼ��顣 �����ǵ�ľ����ȼ��1�֣���˵���ֽ�����������к�O2��
��5��22.4%
���������������1������������֪�IJ����ƶϣ��������Ϊ��Ԫ��ֻ��+3�ۣ���2������ʵ������ƶϣ�Fe(NO3)2���ȷֽ�����Ĺ������ֻ��Fe2O3��Fe2O3������ϡ���ᷴӦ����Fe3+����Һ����Fe3+��KSCN��Һ��죬����ʹ���Ը��������Һ��ɫ����Fe2O3��ϵ��Ϊ1����������������ԭ�Ӹ����غ���ƽ���÷�ӦΪ2Fe(NO3)2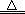 Fe2O3+4NO2��+1/2O2����ϵ���ӱ��ɵã�4Fe(NO3)2
Fe2O3+4NO2��+1/2O2����ϵ���ӱ��ɵã�4Fe(NO3)2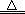 2Fe2O3+8NO2��+O2������4��Fe(NO3)2���ȷֽ��������������У�NO2�Ǻ���ɫ���壬O2����ɫ��ζ���壬�к���ɫ�������������˵���ֽ�����������к���NO2������NO2�ж���������ˮ��Ӧ��������O2��Ӧ��NO����˲���2��Ӧ�����ù���4mol/LNaOH��Һ���ն����NO2������Ũ�����������һ���������ô����ǵ�ľ�����飬��������ľ����ȼ��˵���ֽ�����������к���O2����5�����������֪��������m(Fe2O3)=3.2g��������Ԫ���غ�ɵù�ϵʽ��2Fe��Fe2O3����ԭ����m(Fe)= m(Fe2O3)��2��56/160=3.2g��2��56/160�����ڻ����������Ϊ10g������������Ԫ�ص���������Ϊ3.2g��2��56/160��10g��100%=22.4%��
2Fe2O3+8NO2��+O2������4��Fe(NO3)2���ȷֽ��������������У�NO2�Ǻ���ɫ���壬O2����ɫ��ζ���壬�к���ɫ�������������˵���ֽ�����������к���NO2������NO2�ж���������ˮ��Ӧ��������O2��Ӧ��NO����˲���2��Ӧ�����ù���4mol/LNaOH��Һ���ն����NO2������Ũ�����������һ���������ô����ǵ�ľ�����飬��������ľ����ȼ��˵���ֽ�����������к���O2����5�����������֪��������m(Fe2O3)=3.2g��������Ԫ���غ�ɵù�ϵʽ��2Fe��Fe2O3����ԭ����m(Fe)= m(Fe2O3)��2��56/160=3.2g��2��56/160�����ڻ����������Ϊ10g������������Ԫ�ص���������Ϊ3.2g��2��56/160��10g��100%=22.4%��
���㣺����̽��ʵ�鷽������ƺͶ���ʵ��ļ��㣬�漰������롢��֤���롢���ʵ�鷽������ƽ��ѧ����ʽ�����������Ԫ�����������IJⶨ��֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij̽��С�������ͼ��ʾװ�ý���Fe����ˮ�����ķ�Ӧ��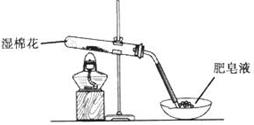
��1��ʵ��ǰ���װ�������Եķ���Ϊ________________________________________________________��
��2������ʵ�������������ʵ�������_____________________________________________��
��3����̽��С���Ϊ���飬����ͼװ�ý��жԱ�ʵ�飬�����þƾ���ơ������þƾ��Ƽ��ȣ���Ӧ�����Ϊ��ɫ��ĩ(������)������ֱ��ò����������ʵ�顣
| ���� | ���� | �������� | �������� |
| 1 | ȡ��ɫ��ĩ����ϡ���� | �ܽ⣬������ | �ܽ⣬������ |
| 2 | ȡ����1����Һ���μ�����KMnO4��Һ | ��ɫ��ȥ | ��ɫ��ȥ |
| 3 | ȡ����1����Һ���μ�KSCN��Һ | ��� | ������ |
| 4 | ����3��Һ�еμ�������ˮ | ��ɫ��ȥ | �ȱ�죬����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�ⶨNa2CO3��NaHCO3����������Na2CO3��������������ȡһ����������Ʒ����ͬѧ����ͼI��ʾװ�ò�������CO2���������ͬѧ����ͼII��ʾװ��ͨ������ܵ����ز�������CO2����������֪����ϡ�����������
��l��ʢ��ϡ�������������Ϊ ��
��2���Լ�XΪ ���Լ�YΪ ��
��3����ͬѧ�ڽ���ʵ��ʱ��Ϊ��С��Ӧע��������У���ѡ����ĸ�� ��
A������ǰӦʹ����װ����ȴ������
B������Z�ĸ߶�ʹ����װ������Һ����ƽ
C������ʱ������Z�ڰ�Һ����͵�����
D������ǰӦͨ��һ������N2ʹ���ɵ�CO2ȫ����������װ��
��4������ͬѧ��ʵ�鷽������ʵ�飬ʹ��õ�Na2CO3����������ƫ�ߵ������У�дһ�֣�
��ʹ��õ�Na2CO3����������ƫ�͵�������
��дһ�֣� ��
��5��Ϊ�����ͬ�IJⶨ��������ʵ�鷽�����ܴﵽʵ��Ŀ�ĵ��� ����ѡ����ĸ����
A��ȡmg�����������Ba��OH��2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɵ�ng����
B��ȡmg����������������ַ�Ӧ������Һ���ȡ����ɡ����յ�ng����
C��ȡmg������ּ��ȣ�������������ng
D����ͼIIװ���е�ϡ�����Ϊϡ�������ʵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ӹ�ҵ����30����FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣�ϸ�ʴҺ���д���CuCl2��FeCl2��FeCl3�������ŷŽ����»�����Ⱦ����Դ���˷ѣ�Ӧ���ǻ������á�������������ʵ���ҽ���ʵ�飺�ӷ�Һ�л���ͭ���������Ļ�����ȫ��ת��ΪFeCl3��Һ����Ϊ��ʴҺԭ��ѭ��ʹ�á�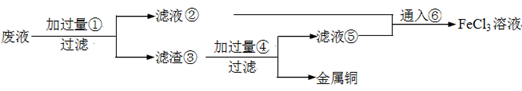
��1��д��FeCl3��Һ��ͭ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2������ϸ�ʴҺ�к���Fe3+��ʵ�������
��3�������ˡ��õ��IJ��������У���ͨ©���� ��
��4����Һ�м�������ٺ�����Ӧ�����ӷ���ʽ��
��5������������ȡ��Һ200 mL�����к�CuCl2 1.5 mol��L��1��FeCl2 3.0 mol��L��1��FeCl3 1.0 mol��L��1����Ҫ��ͭȫ�����գ������Fe�۵�����Ӧ������_____________g�������Ļ�����ȫ��ת��ΪFeCl3��Һ��ͨ��Cl2�����ʵ���������_______________mol��
��6��ij��ѧ��ȤС����������ͼװ����ȡ������ͨ�뵽FeCl2��Һ�л��FeCl3��Һ��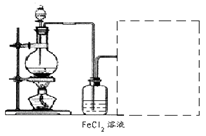
�Ʊ�Cl2�Ļ�ѧ����ʽΪ��
��װ�ò������������������߿��ڻ�����ȱ���֣�����ע�Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ�������Һ�еμ����軯����Һ���ٵμ�˫��ˮ����������Ѫ��ɫ�����ɫ����ȥ�ʻ�ɫ���������ݲ�������Ը�ʵ������ijʵ��С���ͬѧ������̽��
һ���������
����A��������H2O2�ֽ������O2
����B��������KSCN������ΪN2��SO2��CO2��
����C����ɫ��ȥ��ԭ����KSCN������������������
����ʵ��̽��
̽��1
| ʵ����� | ʵ������ | ���� |
| ��1mL 0.1mol/L��FeCl2��Һ�м�2��KSCN��Һ | ��Һ����� | Fe2+��SCN-����� |
| ����ٵ���Һ�м�3%��H2O21�β��� | ��������Ѫ��ɫ ����ɫ | ����H2O2����Һ�� ������ (���ӷ���) |
| ����ڵ���Һ�м�����H2O2��Һ | ��Һ�г��ִ������� Ѫ��ɫ��ȥ | |
| ���ô����ǵ�ľ��������е����� | ľ����ȼ | ����� ���� |
| ʵ����� | ʵ������ | ���� |
| ��ȡ2ml KSCN��Һ�����м��뼸��BaCl2��Һ��ϡ���� | ���������� | |
| ��������õ���Һ�еμ�3%��H2O2 | ��Һ�г��ְ�ɫ���������������� | ��ɫ����ΪBaSO4 |
| �۽�6%��H2O2��Һ����KSCN�����У����ɵ���������ͨ��Ʒ����Һ������KMnO4��Һ�ͳ����ʯ��ˮ | | KSCN��H2O2����������SO2��CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��ʵ�����ö������̸�Ũ���ᷴӦ��ȡ���������ӷ���ʽΪ ��
��2����������dz����������������������£�MnO4������ԭ��Mn2+���ø�����ظ�Ũ���ᷴӦ�������������������ӷ���ʽΪ ��
��3����ʷ�����á��ؿ���������������һ��������CuCl2����������450�����ÿ����е��������Ȼ��ⷴӦ����������Ӧ�Ļ�ѧ����ʽΪ ��
��4������100 mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
�� ������һ���м���10 mL 4 mol/L�İ�ˮ��ǡ����ȫ����������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������1 mol/L NaOH��Һ��10 mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
�� ����һ���м���a mL 1 mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������һ����Ҫ�����ϣ���ѧʽΪFe2O3��xH2O���㷺����Ϳ�ϡ������ϡ��Ľ���Ʒ�ȹ�ҵ��ʵ����ģ�ҵ��������������Fe2O3��������CaO��MgO�ȣ��ͻ�����ۣ���Ҫ�ɷ�ΪFeS2���Ʊ����Ƶ��������£�
��1����������������ж��õ��������������������ֲ����е����÷ֱ��� �� ��
��2���Լ�a���ѡ�� ����ѡ��ʹ�õ��У����ۡ�������ŨHNO3������������ ��
��3���������������õ�����������װ�ÿ�����ʵ�����ư������� ������ţ���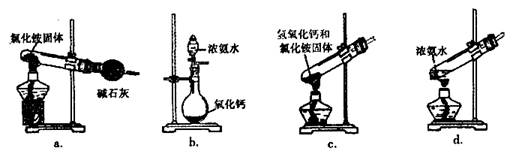
��4��������ҺZ�к���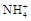 �ķ����� ��
�ķ����� ��
��5����������֪���ڲ�ͬ�¶���Fe2O3��CO��ԭ���������ΪFe3O4��FeO��Fe�����������뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ��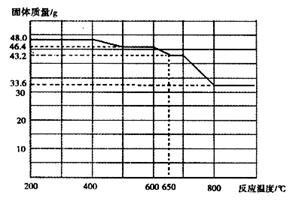
����ͼ���ƶ�670��ʱFe2O3��ԭ����Ļ�ѧʽΪ �������һ����ʵ�飬֤���û�ԭ����ijɷ֣�����ʵ�����������ͽ��ۣ��� ��������ѡ���ɹ�ѡ����Լ���ϡH2SO4��ϡ���ᡢH2O2��Һ��NaOH��Һ��KSCN��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
úȼ�յ������к���CO��SO2���壬���й��������������˵����ȷ����
| A�����߶�������ˮ |
| B�����߶����ж����� |
| C�����߶���ʹƷ����Һ��ɫ |
| D�����߶����γ��������Ҫԭ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com