
BaCl2��xH2O�нᾧˮ��Ŀ��ͨ����������ȷ����
�ٳ�ȡ1.222 g��Ʒ������С�ձ��У���������ϡ���ᣬ�����ܽ⣬�߽���ߵμ�ϡ���ᵽ������ȫ�����ã�
�ڹ��˲�ϴ�ӳ�����
�۽�ʢ�г�������ֽ����ɲ��������գ�ת�����¯�У��������������أ��Ƶó�������Ϊ1.165 g��
�ش��������⣺
(1)�ڲ������У���Ҫ�Ⱥ���ϡ�����________ϴ�ӳ���������������������Ƿ�ϴ���ķ�����__________________________________________________________��
(2)����BaCl2��xH2O�е�x��________(Ҫ��д���������)��
(3)�������У����������������¶ȹ��ߣ����ܻ��в��ֳ�������ֽ�е�̼��ԭΪBaS����ʹx�IJⶨ���________(�ƫ�͡���ƫ�ߡ����䡱)��
(1)����ˮ��ȡˮϴҺ���Թ��У�����ϡ�����ữ���μ�AgNO3��Һ�����ް�ɫ���dz��֣������Cl���Ѿ�ϴ����
(2)��Ʒ��BaCl2�����ʵ���Ϊn(BaCl2)��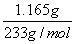 ��5.000��10��3 mol
��5.000��10��3 mol
m(BaCl2)��5.000��10��3 mol��208 g/mol��1.040 g
n(H2O)��n(BaCl2)��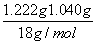 ��(5.000��10��3 mol)��2.02��2
��(5.000��10��3 mol)��2.02��2
(3)ƫ��
[����] (1)ϴ�ӳ���Ҫ������ˮ��������ϴ�Ӻ�ϴ��Һ�в���Cl�����Ѿ�ϴ����(3)�����Ѿ��������ᱵ����ԭΪBaS�������������С��������һ������ô����õ�ˮ������ƫ��x���ݽ�ƫ�ߡ�


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£� �� 3 mol SO2 �� 1 mol O2 ����һ�����ܱ������У� �ڴ��������½������з�Ӧ: 2SO2��g�� + O2��g��  2SO3��g���� ���ﵽƽ��״̬ʱ�� ����˵������ȷ���ǣ� ��
2SO3��g���� ���ﵽƽ��״̬ʱ�� ����˵������ȷ���ǣ� ��
A������ SO3 Ϊ2 mol B��SO2 �� SO3 ���ʵ���֮��һ��Ϊ 3 mol
C������SO2 �����ʵ�������O2������ D��SO2 �����ʵ�����SO3 ���ʵ���һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��80 g�ܶ�Ϊd g��cm��3����������Һ�У�����2.8 g Fe3���������Һ��SO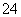 �����ʵ���Ũ��Ϊ(��λΪmol��L��1)
�����ʵ���Ũ��Ϊ(��λΪmol��L��1)
A��15d/16 B��5d/16 C��3d/8 D��5d/8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����±��ṩ�IJ����������Dz���������ѡ������ʵ����Ӧʵ��Ŀ�ĵ���
| ѡ�� | ʵ��Ŀ�� | �������� |
| A | �����Ҵ������������Ļ���� | ��Һ©�����ձ� |
| B | ��pH=1����������100ml, pH=2������ | 100mL����ƿ���ձ�������������ͷ�ι� |
| C | ����ˮ������-KI��Һ�Ƚ�Br2��I2��������ǿ�� | �Թܡ���ͷ�ι� |
| D | ��NH4Cl��Ca��OH��2�����Ʊ����ռ�NH3 | �ƾ��ơ��ձ������ܡ�����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤��������ֵ������˵����ȷ����(����)
A�������£�0.2 mol Fe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3 NA
B�������£�1 L pH��13��NaOH��Һ�У���ˮ�����OH����ĿΪ0.1NA
C������ȼ�ϵ����������22.4 L(��״��)����ʱ����·��ͨ���ĵ�����ĿΪ2NA
D��5NH4NO3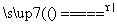 2HNO3��4N2����9H2O��Ӧ�У�����28 g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
2HNO3��4N2����9H2O��Ӧ�У�����28 g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���

��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)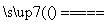 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O ��I2===S4O
��I2===S4O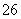 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
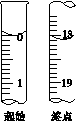
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤����������ֵ������˵������ȷ����(����)
A��1.8 g��ˮ(D2O)�к��е��������͵�������Ϊ1.0NA
B��0 �桢1��������ѹ�£�22.4 L NO ��22.4 L O2��Ϻ����������еķ�������Ϊ1.5NA
C����4 mol Si��O���Ķ������辧���У���ԭ����Ϊ2NA
D����11.2 L Cl2ͨ��������ʯ�������Ʊ�Ư�ۣ�ת�Ƶĵ�����Ϊ0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(1)A�����ڱ��е�λ����________��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ��__________________________��
(2)B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ__________����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________���ڲ����м�������KI����Ӧ�����CCl4�����л�����______ɫ��
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ������A�Ķ�Ԫ���ӻ����� |
| b | �����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ����ѧ���ΪBDF2 |
| d | ��ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������________��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________�����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á�
��ͼK103��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)��
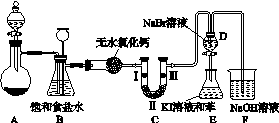
ͼK103
(1)�Ʊ�����ѡ�õ�ҩƷΪ������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(2)װ��B��������________________________��ʵ�����ʱC�п��ܷ�����������д����������ʱB�е�����______________________________________��
(3)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���________(ѡ��a����b����c��)��
| a | b | c | |
| �� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(4)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������D�е�������Һ����E�У���E���۲쵽��������________________________________��������________(��ܡ����ܡ�)˵����ķǽ�����ǿ�ڵ⣬ԭ����__________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com