
�������ʵ�����A��B�����2L���ܱ������У�������Ӧ��3A(g)+B(g)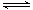 xC(g)+2D(g)
��H<0��5min��ﵽƽ�⣬ƽ��ʱ���D��Ũ��Ϊ0.5mol/L��C(A)��C(B)=1��2��C��ƽ����Ӧ����Ϊ0.05mol/(L��min)
xC(g)+2D(g)
��H<0��5min��ﵽƽ�⣬ƽ��ʱ���D��Ũ��Ϊ0.5mol/L��C(A)��C(B)=1��2��C��ƽ����Ӧ����Ϊ0.05mol/(L��min)
(l)x��ֵΪ_______________;
(2)A��ƽ��Ũ��____________;
(3)�����º��ݣ���������ƽ��ʱ��ѹǿ����ʼʱ��ѹǿ��___________;
(4)��ͼ��ijһʱ����и÷�Ӧ�����뷴Ӧ���̵����߹�ϵͼ���ش��������⣺
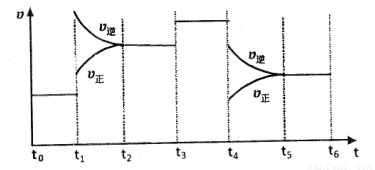
�ٴ���ƽ��״̬��ʱ�����____________��
�� ��
�� ��
�� ʱ����ϵ�зֱ���ʲô���������˱仯��
ʱ����ϵ�зֱ���ʲô���������˱仯��
 ___________��
___________�� _____________ ��
_____________ �� _____________����A-E��
_____________����A-E��
A������ B������ C���Ӵ��� D����ѹ E����ѹ
�����и�ʱ���ʱ��A�����������ߵ���____
A.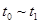 B��
B��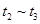 C��
C�� D��
D��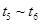
��1��1 ��2��0.5mol/L ��3��9:10 ��4����t0��t1 t2��t3 t3��t4 t5 --t6 ��A��C ��E ��D
��������
�����������1��V(C)=��C/��t���Ԧ�C= V(C)����t=0.05mol/(L��min) ��5min=0.25 mol/L���ڷ�Ӧ��C(C):C(D)=X:2 0.25: 0.5= X:2������X=1.��2����C��A��:��C(D)=3:2 ��C��A��: 0.5=3:2. ��C��A��=0.75�����迪ʼʱ�����A��B�����ʵ���Ϊ2m,����ʼʱA��B�����ʵ���Ũ��Ϊm mol/L.���ݷ�Ӧ�仯��A Ũ��Ϊ0.75 mol/L�ͷ�Ӧ����ʽ��A��B��ϵ����ϵ��֪��Ӧ�仯��BŨ��Ϊ0.25 mol/L������ƽ��Ũ��C(A)=(m-0.75) mol/L ,C(B)= (m-0.25) mol/L. ��ΪC(A): C(B)=1:2����(m-0.75) :(m-0.25) =1:2,���m=1.25. ����A��ƽ��Ũ��Ϊ��C(A)=(m-0.75) mol/L=(1. 25-0.75) mol/L=0. 5mol/L����3����ʼʱA��B��������ʵ���Ϊ2.5 mol,����������ʵ���Ϊ5 mol��ƽ��ʱ������������ʵ���Ϊn(A)= 0. 5mol/L��2L=1mol; n (B)= (m-0.25) mol/L��2L= (1.25-0.25) mol/L��2L =2mol;n(C)= 0.25 mol/L��2L =0. 5mol/L ;n(D)= 0.5mol/L��2L =1mol.ƽ��ʱ����������ʵ���Ϊ��1+2+0. 5+1��mol=4.5mol����n(ƽ��)��n(��ʼ)= 4.5��5=9:10.���ݰ���٤�����ɵ���������ͬ����ͬ������ܱ������У������ѹǿ�ȵ������ǵ����ʵ���֮�ȡ�����P(ƽ��)��P(��ʼ)= n(ƽ��)��n(��ʼ)= 9:10.��4���ٿ��淴Ӧ��ƽ��ʱ����Ӧ���淴Ӧ��������ȡ���ͼ��֪ƽ��ʱ���Ϊ��t0��t1 t2��t3 t3��t4 t5 --t6 ����t1ʱ������Ӧ���淴Ӧ�����ʶ�������V(��)����V(��)��˵����Ӧ���ʼӿ죬ƽ�������ƶ��������������¶ȡ�ѡA����t3ʱ����Ӧ���淴Ӧ�����ʶ�������V(��)����V(��)��˵����Ӧ���ʼӿ죬ƽ�ⲻ�ƶ��������Ǽ��������ѡ��Ϊ��C ��t4ʱ����Ӧ���淴Ӧ�����ʶ���С����V(��)����V(��)˵����Ӧ���ʼ�����ƽ�������ƶ������ܵı仯�Ǽ�Сѹǿ��ѡ��ΪE������ʹA�����������ߣ���ƽ�������ƶ����ŷ���Ҫ��t0��t1ƽ��״̬�� t2��t3 ƽ�������ƶ��ﵽ�µ�ƽ�⣬A������ǰһ�θߣ� t3��t4ƽ��û�ƶ�����t2��t3����ͬ�� t5 --t6ƽ�������ƶ����ﵽ�µ�ƽ�⡣��A������ߵ�Ϊt5 --t6ѡ��Ϊ��D��
���㣺������������Ի�ѧƽ���Ӱ�켰��Ӧ����ʽ�и����ʵ����ʹ�ϵ��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�������
��8�֣��������ʵ�����A��B�����1 L���ܱ������У��������·�Ӧ
3A(g)��B(g) xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
(1)��ʱA��Ũ��c(A)��________mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
(2)B��ƽ����Ӧ����v(B)��________mol/(L��min)��
(3)x��ֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
��8�֣��������ʵ�����A��B�����1 L���ܱ������У��������·�Ӧ
3A(g)��B(g)  xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
(1)��ʱA��Ũ��c(A)��________mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)��________mol��
(2)B��ƽ����Ӧ����v(B)��________mol/(L��min)��
(3)x��ֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ��һ��ѧ�ڵ����ζο���ѧ�Ծ� ���ͣ������
(4��)�������ʵ�����A��B�����2 L���ܱ������У��������·�Ӧ3A(g)��B(g) xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
(1)��ʱA��Ũ��c(A)�� mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)��n(B)�� mol��
(2)B��ƽ����Ӧ����v(B)�� mol/(L��min)�� (3)x��ֵΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com