
��A��B��C��D��E��F�������ʣ�����֮���ת����ϵ��ͼ��ʾ(���������ֲ���δ���)��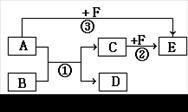
��1����A��DΪ���壬����ʹ�����ʯ��ˮ����ǣ�B��F����ɫ��Ӧ������ɫ�ܲ����۲�Ϊ��ɫ�� B��CΪ���Σ�F����ҺΪ�Ϻ�ɫ����C��F��������Һ�з�����Ӧ�ڵ����ӷ���ʽΪ ��
��2����1 mol A�����к���3 mol���Թ��ۼ��� B��C��F���Ƕ�����Ԫ����ɵķǽ������ʣ������£�ֻ��DΪ���壬����Ϊ���塣��Ӧ�۵Ļ�ѧ����ʽΪ ��
ijͬѧ�������ͼ��ʾװ�ã��г�����ʡ�ԣ���������(2)������B�Ļ�ѧ����̽����
������Ϊ��װ������Ƿ����������������θĽ��� ��(���������ʲ���)
�ڢ��в����������� ��
�۷�Ӧ�����Ӻ�����п��ܺ��еĽ��������ӵ�ʵ������� ��
��ͨ������̽��������B����Ҫ��ѧ������ ��
(12��)��1��5SO32-��2MnO4-��6H+��2Mn2+��5SO42-��3H2O ���������� (2��)
��2��4NH3��5O2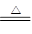 4NO + 6 H2O �������������� (2��)
4NO + 6 H2O �������������� (2��)
�ٲ�������Ӧ�ڢ�֮����ϳ�ȥHCl��װ�á� �������������� (2��)
��ʯ����Һ�ȱ�����ɫ ���������� (2��)
��ȡ��Һ�����ֱ������֧�Թ��У�������һֻ�μ����軯����Һ������Һ��죬����Fe3+��
����һֻ�Թ��еμ����軯����Һ����������ɫ����������Fe2+��(ÿ������1�֣���2��)
��ǿ�����ԣ�����ˮ����Ӧ��ˮ��Һ�������Ժ�����(Ư��)�� ��2�֣���3��ȫ�֣�
���������������1����A��DΪ���壬����ʹ�����ʯ��ˮ����ǣ���˵��������CO2��SO2��B��F����ɫ��Ӧ������ɫ�ܲ����۲�Ϊ��ɫ��˵�����м�Ԫ�ء� F����ҺΪ�Ϻ�ɫ����˵��F�Ǹ�����ء�����F�ܺ�A��Ӧ��˵��A�Ǿ��л�ԭ�Եģ�����A��SO2��D��CO2��B��CΪ���Σ�����B��̼��أ�C��������ء���C��F��������Һ�з�����Ӧ�ڵ����ӷ���ʽΪ5SO32-��2MnO4-��6H+��2Mn2+��5SO42-��3H2O��
��2����1 mol A�����к���3 mol���Թ��ۼ������A�ǰ�����B��C��F���Ƕ�����Ԫ����ɵķǽ������ʣ������£�ֻ��DΪ���壬����Ϊ���壬��˵��BӦ����������C�ǵ�����D���Ȼ�泥�F��������E��NO����Ӧ�۵Ļ�ѧ����ʽΪ4NH3��5O2 4NO + 6 H2O��
4NO + 6 H2O��
���������Ʊ�����ʱ��Ũ������лӷ��ԣ��������ɵ������к����Ȼ��⣬����ź�����ʵ�飬����Dz�������Ӧ�ڢ�֮����ϳ�ȥHCl��װ�ã������ñ��͵�ʳ��ˮ��ȥ�Ȼ��⡣
����������ˮ��������ʹ����ᣬ�������Ժ�ǿ�����ԣ����Ԣ��в�����������ʯ����Һ�ȱ�����ɫ��
����������ǿ�����ԣ��ܰ��Ȼ��������������Ȼ��������Է�Ӧ�����Ӻ�����п��ܺ��еĽ��������ӵ�ʵ�������ȡ��Һ�����ֱ������֧�Թ��У�������һֻ�μ����軯����Һ������Һ��죬����Fe3+������һֻ�Թ��еμ����軯����Һ����������ɫ����������Fe2+��
�ܸ������Ϸ�����֪����������Ҫ������ǿ�����ԣ�����ˮ����Ӧ��ˮ��Һ�������Ժ�����(Ư��)�ԡ�
���㣺��������ͼ����жϡ������Ʊ��Լ����ʵļ����ʵ��̽��
�������������е��Ѷȵ����⣬�����ۺ���ǿ����ѧ����˼ά����Ҫ��ߣ�����������ѧ���������������ͷ�ɢ˼ά������Ҳ����������ѧ���淶���Ͻ���ʵ�����������������ѵ��ǿ�ͼ����жϣ���Ҫע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϡ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼװ�ÿ������ռ����岢��֤�仯ѧ���ʣ����ж�Ӧ��ϵ��ȫ��ȷ����
| | ���� | �Լ� | ���� | ���� |
| A | NO | ��ɫʯ����Һ | ��Һ��� | NO��ˮ��Ӧ�������� |
| B | Cl2 | NaBr��Һ | ��Һ�ʳȻ�ɫ | �ȵķǽ����Դ����� |
| C | SO2 | ����KMnO4��Һ | ��Һ��ɫ | SO2��Ư���� |
| D | NH3 | MgCl2��Һ | ������ɫ���� | NH3�м��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼��ƺ�����ƶ��ǸƵ���Ҫ��������������������ж����Ź㷺��Ӧ�á��ס�������ͬѧ�ֱ��̼��Ƶ��Ʊ�������Ƶ����ʽ���������̽����������벢��ɶ��й�����Ľ��
��1������ʹ�ô���ʯ(��������Fe2O3����)�������Ʊ�̼��Ƶ�ʵ���������£�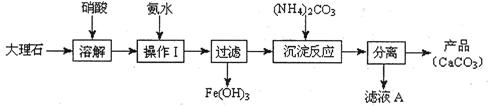
���ܽ����ʯʱ������������������ԭ���� ��
�����������У������롱�ò�Ʒ��������ʵ���������Ϊ�����ˡ� �� ��
�ۡ���ҺA���г�H+�����⣬�����е��������� ������������ӵ�ʵ�鷽���ǣ�ȡ������ҺA�� ���Թ��л�ϡ����ȳ�ַ�Ӧ����ʪ��ĺ�ɫʯ����ֽ(��pH��ֽ)�����Թܿڣ��۲����ɡ�
��2�������ij����ƾ���(xCaS04��yH20)���ȷֽ���йط�Ӧ����̽��������ȡ6.52g�þ�����м��ȣ����ȹ����У�����������ʱ��ı仯�������ͼ��ʾ����֪t5��t6ʱ����ڹ������������ԭ���Dz������������壬��Ӧ�Ļ�ѧ����ʽΪ��2CasO4 2CaO+2S02��+O2����
2CaO+2S02��+O2����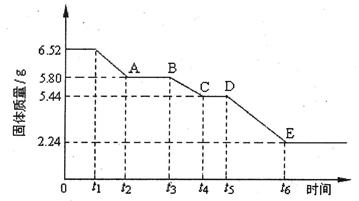
�ټ���ʱ���þ��忪ʼ������ѧ�仯��ʱ���� (�t1������t3����t5��)��
��t4��t5ʱ��ι���Ļ�ѧʽΪ ��
��tl��t2ʱ��ι��巢����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�м���ʵ��С���ͬѧ��������ͼװ�ý��С�һ�����á���ʵ��̽��(a��ʢ�ŵ�Һ��������ٵ��£�b��ʢ�ŵ�ҩƷ����������c��d��ʢװҺ�壬���ܾ�����Һ������)��ÿ��ͬѧ������a��b��c��d�зֱ�ʢ�Ų�ͬ���ʣ�����ȡij�����岢���������ʡ�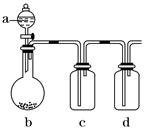
����ش����¸���ͬѧ�ڽ���ʵ����Ʒ�������������⣺
��.��1����a��Ũ���b���������(�������Աȶ�������ǿ�ܶ�)��c����ɫ�ʻ��ꡣ��ʵ�������c�е�����_____________________________________________________________��
dװ����ʢ��ҩƷ��������______________________________����д��d�з�Ӧ�����ӷ���ʽ�� ___________________________________________________________________��
��2����a��ϡ���b�����Ƿۣ�c������̼������Һ��d������Na2SiO3��Һ����ʵ���
���У�c�г��ֵ�������____________________��d��������____________����˵��
________________________________________________________________________��
��.����Ϊ����ͬѧ����ȡ����֮ǰ��Ӧ���еIJ�����________________���㻹�������ô�װ�õ�a��b��������ȡ��������(ֻдһ��)_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ѧʽ��ʾΪMgCO3��CaCO3��Ϊԭ���Ʊ�Mg(OH)2�Ĺ�����������ͼ��ʾ��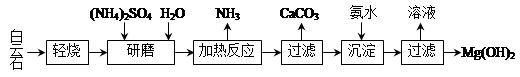
��1����ĥ�������� ��
��2���ù����п�ѭ��ʹ�õ������� �� ��д��ѧʽ����
��3������ʯ���յ���Ҫ������MgO��CaCO3������ͳ�����ǽ�����ʯ���ȷֽ�ΪMgO��CaO����ȡ������ʯ���յ��ŵ��� ��
��4�����ȷ�Ӧ�����ӷ���ʽΪ ��
��5���ټ��ȷ�Ӧʱ����323k��353k��Һ��c(NH4+)�뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ��������ͼ����373k�����ߡ�
����ͼ��֪�������¶����ߣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
Ϊ̽����ҵ����������ͭ�Ͻ���ϵ������ã���ͬѧ��Ƶ�ʵ�鷽�����£�
��ش�
��1���̷��Ļ�ѧʽΪ ��
��2��д����Ӧ�ٵĻ�ѧ����ʽ ��
��Ӧ�����ɳ��������ӷ�Ӧ����ʽ ��
��3��Ϊ�˼����ҺD�к��еĽ������ӣ������ʵ�鷽��Ϊ���Լ���ѡ���� ��
��4��������B�еμ�ϡ����ʱ�����ַ�Ӧ���ʱ�һ������۷�ӦҪ�죬��ԭ���� ��
��5����������ɫ��ѧ���գ�������E�м���ϡ������Լ�Y�Ƶ������壬�Լ�YΪ��ɫҺ�壬��Ӧ�ܵ��ܻ�ѧ����ʽΪ ������������ɫ��ѧ���գ���ѡ�Լ�YΪ1mol/L�����ᣬ��ʹ3molCuȫ���ܽ�����Һ�к�ͭԪ�ص����ʽ�ΪCuSO4��������������� L��
��ʯ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ѱۣ���Ҫ�ɷ���TiO2�����㷺�������ᡢ���ϡ���ֽ����ҵ�����������Ҵ���ˮ������Ĵ�������ͼ������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ�������Ѱ۲���ø���ƷFeSO4��7H2O�Ĺ�������ͼ��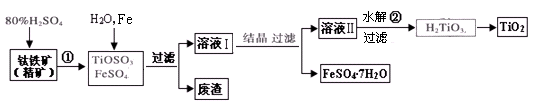
��1�������������ᷢ����Ӧ�ٵĻ�ѧ����ʽΪ ����TiOSO4��FeSO4��Һ�м���Fe��Ŀ���� ��
��2����Һ����TiOSO4�ڼ��������·���ˮ�ⷴӦ�ڵ����ӷ���ʽΪ ���ɻ������õ������� ��
��3��Ϊ�ⶨ��Һ����TiOSO4�ĺ���������ȡ������Һ10 mL��ˮϡ����100 mL���ӹ������ۣ������ʹ����ȫ��Ӧ��3TiO2+ +Al+6H+=3Ti3++Al3++3H2O�����˺�ȡ����Һ20.00 mL�������еμ�2��3��KSCN��Һ��ָʾ������ ����һ�ֲ������������ƣ��μ�0.1000mol��L��1 FeCl3��Һ������Һ���ֺ�ɫ�ﵽ�ζ��յ㣬��ȥ��30.00mL FeC13��Һ��������Һ��TiOSO4�����ʵ���Ũ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
[ʵ�黯ѧ]
��������������ҽҩ���й���ϵȹ�ҵ��Ӧ�ù㷺���Զ������ױ���Ϊԭ����ȡ������������(��ɫ�ᾧ���۵�242 �棬�е�Լ359 �棬����ˮ����������)�ķ�Ӧԭ��Ϊ��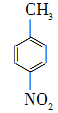 ��Na2Cr2O7��4H2SO4�D��
��Na2Cr2O7��4H2SO4�D��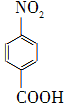 ��Na2SO4��Cr2(SO4)3��5H2O
��Na2SO4��Cr2(SO4)3��5H2O
ijС���Զ������ױ�������Ϊԭ����ȡ�������������ʵ��װ������ͼ��ʾ(���Ⱥ������̶�װ�þ�����ȥ)��ʵ�鲽�����£�
����1����250 mL������ƿ�����μ��������Ķ������ױ����ظ����Ʒ�ĩ��ˮ������ֻ�ϡ��ڽ����£��õ�Һ©����������Ũ�������0��5 h����ӦҺ�ʺ�ɫ��
����2������Ӧ�������ȴ��������ˮ��ֻ�ϣ����˲���50 mLˮ������ϴ�ӡ���ϴ�Ӻ�Ĺ������ʢ������5%������Һ�У�ˮԡ����10 min����ȴ����ˡ�
����3�������˺�Ĺ�����������5%NaOH��Һ�У�50 �����Ⱥ���ˣ�����Һ�м�����������̿����к���ȳ��ˡ����õ�����Һ�������뵽ʢ������15%������Һ���ձ��У�������ɫ���������ˣ���ˮϴ�ӣ�����ôֲ�Ʒ����
��1�� �ڲ���1�С����衱���õ綯��������ɵģ����������ĺô���________��________��
��2�� ��������ƿ�У�����Ũ����ļ��룬��Ӧ�¶�Ѹ��������Ϊʹ��Ӧ�¶Ȳ��¹��ߣ���Ҫʱ�ɲ�ȡ�Ĵ�ʩ��________��
��3�� �ڲ���2�У��������ù������Ҫ�ɷ���________������װ������������������ѹϵͳ�⣬����________��________(����������)��
��4�� ����3��NaOH��Һ���д�����������Ҫ��____��
��NaOH��Һ��������50 �����Ⱥ���˵�ԭ����____��
��5�� �ƵõĴֲ�Ʒ��Ҫ��һ������(����)�����ݶ�������������й����ʿ�֪��������о���(����)�������Ҵ���Һ��ɣ�Ҳ���Բ���________����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(15��)��֪��BaSO4(s)+4C(s) ����4CO(g)+BaS(s)
��ҵ�����ؾ�ʯ��(��Ҫ�ɷ�BaSO4��������Fe2O3��SiO2)Ϊԭ�ϣ�ͨ���������������Ȼ�������(BaCl2��nH2O)��
(1)��������������ʵ���ұ����ؾ�ʯ���մ��������������壬ǡ���ķ����� (����ĸ���)��
a���ó���ʯ��ˮ�������� b����Ũ������������ c����ȼ����
(2)�����ؾ�ʯ��ʱ�����ܷ����ĸ���Ӧ�Ļ�ѧ����ʽ�� (д����)��
(3)Ϊ�ⶨ��Ʒ�Ȼ�������(BaCl2��nH2O)�е�nֵ���������ʵ�鲽�裬�벹�����ƿ�ȱ���ʵ�鲽�裺�ٳ�����Ʒ����������Ʒ�������� (����������)����ȴ���� ���ݺ��ز��������ز�����Ŀ���� �������ղ����nֵƫ���ܵ�ʵ��������ԭ���� (����һ�����)��
(4)������װ�����Ҳ�����(3)��ʵ�顣��ѡ������ǡ����װ�����(���������Ⱥͼг�����ʡ��)���������ʵ�飺 (��װ�ô����ԡ�A
 ����
����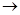 ����ʽ��ʾ��ÿ��װ�ò����ظ�ʹ��)
����ʽ��ʾ��ÿ��װ�ò����ظ�ʹ��)
(5)���ؾ�ʯ����̼���Ȼ��ƹ�ͬ���գ�����ֱ�ӵõ��Ȼ�������Ӧ�Ļ�ѧ����ʽΪ��
BaSO4+4C+CaCl2����4CO +CaS+BaCl2�������Ǵӱ��պ�Ĺ����з���õ��Ȼ��������ʵ���������(��֪�Ʋ�����ˮ������������)�����ڿո��������д�������ơ�
+CaS+BaCl2�������Ǵӱ��պ�Ĺ����з���õ��Ȼ��������ʵ���������(��֪�Ʋ�����ˮ������������)�����ڿո��������д�������ơ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com