
����A�����·�Ӧ·�ߣ�
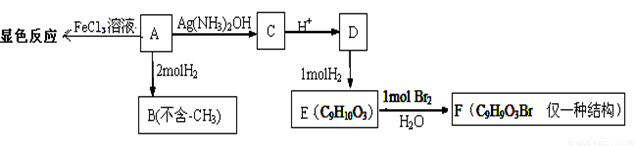
��1��1mol ����A���������H2�����ʵ���Ϊ
��2��A��C�Ļ�ѧ����ʽΪ ��
��3��H��G��B��Ϊͬ���칹��������������������H��G��Ũ��ˮ��NaHCO3������Ӧ���ҽṹ�о�������-CH3�������Ų���ͬʱ����ͬһ��̼ԭ���ϣ�����1molH��G ������2molNa��Ӧ����H����һ��ȡ��ֻ��2�֣�G����һ��ȡ��ֻ��3�֡�H�Ľṹ��ʽΪ ��G��һ�ֽṹ��ʽΪ ��
��4����֪��һ��������R1CH=CHR2 �� R1CHO+ R2CHO��A��һ��������������������X��Y��
X�Ƿ����廯���Y��һ�ֻ�ԭ�ԵĶ�Ԫ���ᡣ
д��X��Ũ���������µ����۷�Ӧ����ʽ ��
��1��5 mol
��2��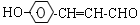 +2Ag��NH3��2OH
+2Ag��NH3��2OH
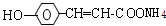 +2Ag��+3NH3+H2O
+2Ag��+3NH3+H2O
��3��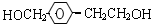
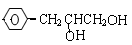 ����
����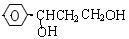 ����
����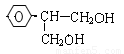 ����дһ���÷֣�
����дһ���÷֣�
��4��n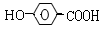
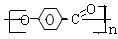 +nH2O
+nH2O
��������
�����������1��A�����Ȼ���������ɫ��Ӧ��˵���б�������������1mol ����A����2mol�����ӳɣ�����1mol ����A�����������5 mol����2��A��������9��̼ԭ�ӣ������б��������ǻ���ȩ��������2 mol�����ӳ��Ҳ�������������A�ĽṹΪ��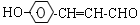 ��
��
���㣺�����л���Ľṹ�����ʡ����۷�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�






 ��W����Һ��ʹpH��ֽ��죬���ܷ���������Ӧ��
��W����Һ��ʹpH��ֽ��죬���ܷ���������Ӧ��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ��ƶ���

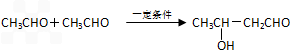
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡģ���� ���ͣ��ƶ���
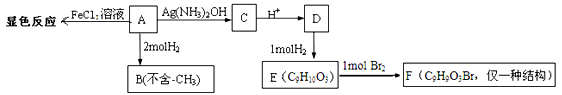
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009-2010ѧ���Ĵ�ʡ�˱����˱���ާϪ��ѧ�߶����£���ĩ��ѧģ���Ծ��������������棩 ���ͣ������
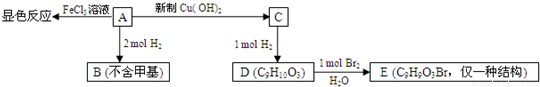
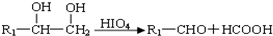
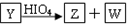 ��W����Һ��ʹpH��ֽ��죬���ܷ���������Ӧ��
��W����Һ��ʹpH��ֽ��죬���ܷ���������Ӧ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com