
ij��ѧС������Ũ����Ͷ��������ڼ�����������ȡ���������������������йص�̽��ʵ�飬��ȡ������װ����ͼI�͢�
��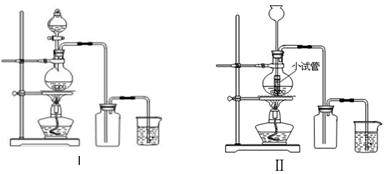
��1����ȡ�����ķ�Ӧ�����ӷ���ʽ ��
��2��װ�â���С�Թܵ�����Ϊ ��
��3��װ��I��װ�â�Ƚϣ�װ��I����Ҫ�ŵ�Ϊ�� ��
��4�������ɵ�����ͨ��ˮ�У����Ƶ���ˮ����ˮ�����ֽ����� ���塣
��5��������ʹʪ��ĺ�ɫ������ɫ������ʹ��ɫ������ɫ�����ʣ�ͬѧ�ǵĿ�����һ�£���Ϊ��ˮ�д��ڵļ������Ӷ��п��ܣ���������ѧʵ���ҳ����Լ������ʵ�飬�ó���ȷ���ۡ�
| ������� | �ռ����� | ������� | ��֤���� | �ó����� |
| ��ˮ�к���������ʹʪ��ĺ�ɫ������ɫ�� | ��Cl2��ǿ������ ��Cl2����ˮ��Ӧ���������HClO ��HClO��ǿ������ | �� �� ������ʹ������ɫ�� �� �� ��H2Oʹ������ɫ | ��֤����٣��Ѻ�ɫ�ɲ��������Cl2�ļ���ƿ����������ɫ�� ��֤����ڣ� �� ��֤����ܣ��Ѻ�ɫ��������ˮ���������ɫ�� | ʹ��ɫ������ɫ�������� �� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ij��ѧС������Ũ����Ͷ��������ڼ�����������ȡ���������������������йص�̽��ʵ�飬��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
A B
��1��д����ȡ������Ӧ�����ӷ���ʽ __________________________��װ����ʹ�÷�Һ©������ʹ�ó���©����ԭ����_______________________________________
_____________________________________________________________________;
ʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬�����A��B֮�䰲װʢ�� �Լ��ľ���װ�á�
��2�����ú���0��2molHCl��MnO2��Ӧ���������Ƶ�Cl2���(��״����)����С��1��12L��ԭ�� ��
��3����֪��H2CO3H++HCO3- Ka1 =4��45��10-7
HCO3-H++CO32- Ka2=5��61��10-11
HClOH++ClO- Ka=2��95��10-8
���������̼��ʹ�����ĵ��볣����д�����������·�����Ӧ�����ӷ���ʽ���ٽ���������ͨ�������̼������Һ��______________________________��
����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ������ λ�ú�����ĸ������һ��װ�ã���װ����Ӧ���� �Լ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɳ��һ�п�ǰԤ�����ѧ���� ���ͣ�ʵ����
��14�֣�ij��ѧС������Ũ����Ͷ��������ڼ�����������ȡ���������������������йص�̽��ʵ�飬��ͬѧ�������ͼ��ʾ��ʵ��װ�á�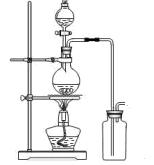
A B
��1��д����ȡ������Ӧ�����ӷ���ʽ __________________________��װ����ʹ�÷�Һ©������ʹ�ó���©����ԭ����_______________________________________
_____________________________________________________________________;
ʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬�����A��B֮�䰲װʢ�� �Լ��ľ���װ�á�
��2�����ú���0��2molHCl��MnO2��Ӧ���������Ƶ�Cl2���(��״����)����С��1��12L��ԭ�� ��
��3����֪��H2CO3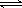 H++HCO3- Ka1 =4��45��10-7
H++HCO3- Ka1 =4��45��10-7
HCO3-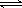 H++CO32- Ka2=5��61��10-11
H++CO32- Ka2=5��61��10-11
HClO H++ClO- Ka=2��95��10-8
H++ClO- Ka=2��95��10-8
���������̼��ʹ�����ĵ��볣����д�����������·�����Ӧ�����ӷ���ʽ���ٽ���������ͨ�������̼������Һ��______________________________��
����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ������ λ�ú�����ĸ������һ��װ�ã���װ����Ӧ���� �Լ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ���ѧ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
ij��ѧС������Ũ����Ͷ��������ڼ�����������ȡ���������������������йص�̽��ʵ�� ����ȡ������װ����ͼI�͢�
����ȡ������װ����ͼI�͢� ��
��
��1����ȡ�����ķ�Ӧ�����ӷ���ʽ ��
��2��װ�â���С�Թܵ�����Ϊ ��
��3��װ��I��װ�â�Ƚϣ�װ��I����Ҫ�ŵ�Ϊ�� ��
��4�������ɵ�����ͨ��ˮ�У����Ƶ���ˮ����ˮ�����ֽ����� ���塣
��5��������ʹʪ��ĺ�ɫ������ɫ������ʹ��ɫ������ɫ�����ʣ�ͬѧ�ǵĿ�����һ�£���Ϊ��ˮ�д��ڵļ������Ӷ��п��ܣ���������ѧʵ���ҳ����Լ������ʵ�飬�ó���ȷ���ۡ�
| ������� | �ռ����� | ������� | ��֤���� | �ó����� |
| ��ˮ�к���������ʹʪ��ĺ�ɫ������ɫ�� | ��Cl2��ǿ������ ��Cl2����ˮ��Ӧ���������HClO ��HClO��ǿ������ | �� �� ������ʹ������ɫ�� �� �� ��H2Oʹ������ɫ | ��֤����٣��Ѻ�ɫ�ɲ��������Cl2�ļ���ƿ����������ɫ�� ��֤����ڣ� �� ��֤����ܣ��Ѻ�ɫ��������ˮ���������ɫ�� | ʹ��ɫ������ɫ�������� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɳ��һ�п�ǰԤ�����ѧ���� ���ͣ�ʵ����
��14�֣�ij��ѧС������Ũ����Ͷ��������ڼ�����������ȡ���������������������йص�̽��ʵ�飬��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
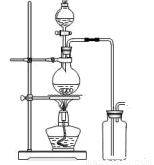
A B
��1��д����ȡ������Ӧ�����ӷ���ʽ __________________________��װ����ʹ�÷�Һ©������ʹ�ó���©����ԭ����_______________________________________
_____________________________________________________________________;
ʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬�����A��B֮�䰲װʢ�� �Լ��ľ���װ�á�
��2�����ú���0��2molHCl��MnO2��Ӧ���������Ƶ�Cl2���(��״����)����С��1��12L��ԭ�� ��
��3����֪��H2CO3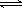 H++HCO3-
Ka1 =4��45��10-7
H++HCO3-
Ka1 =4��45��10-7
HCO3-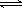 H++CO32-
Ka2=5��61��10-11
H++CO32-
Ka2=5��61��10-11
HClO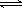 H++ClO-
Ka=2��95��10-8
H++ClO-
Ka=2��95��10-8
���������̼��ʹ�����ĵ��볣����д�����������·�����Ӧ�����ӷ���ʽ���ٽ���������ͨ�������̼������Һ��______________________________��
����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ������ λ�ú�����ĸ������һ��װ�ã���װ����Ӧ���� �Լ��������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com