
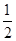 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��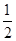 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
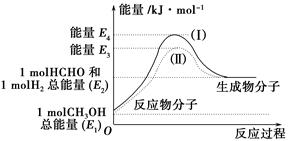
| D����Ӧ�ڵ������仯��ͼ��ʾ |
 HCHO(g)��H2(g)����H����(E2��E1)kJ��mol��1��(2)C
HCHO(g)��H2(g)����H����(E2��E1)kJ��mol��1��(2)C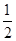 mol O2(g)������������1 mol CO2(g)��2 mol H2(g)������������ͼʾ������D����
mol O2(g)������������1 mol CO2(g)��2 mol H2(g)������������ͼʾ������D����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ӻ������ж��������Ӽ� |
| B�����ӻ������е�������ֻ���ǽ������� |
| C�����ӻ�������������ˮ����ˮ��Һһ�����Ե��� |
| D������ˮ���Ե���Ļ�����һ�������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�� (NH3) (NH3) | B�� �á� �á� H(H2O2) H(H2O2) |
C���� ��(CO2) ��(CO2) | D�� (HClO) (HClO) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | B12�ṹ��Ԫ | SF6���� | S8���� | HCN |
| �ṹģ��ʾ��ͼ | 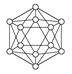 | 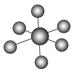 | 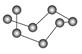 | 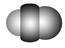 |
| ��ע | �۵�1873 K | �� | ������CS2 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ�� O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1 O2(g)=CO2(g)����H����283.0 kJ��mol��1
O2(g)=CO2(g)����H����283.0 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ABn������Aԭ�ӵ����м۵��Ӷ�����ɼ� |
| B����ABn������A�����ԭ������ӦС��B�����ԭ������ |
| C����ABn������ÿ�����ۼ��ļ�������� |
| D�������в��ܺ�����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CuSO4��5H2O | B��[Fe��SCN��2]Cl2 |
| C��NH4Cl | D��[Ag��NH3��2]OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com