
 ��Ʒ��������Ҫ������
��Ʒ��������Ҫ������  ��
��| �ζ����� | ����Һ��� (mL) | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������߸���������ȵ�ֽƬ������NaOH |
| B��ϴ���ձ��Ͳ���������Һδת������ƿ�� |
| C������ƿ��ԭ����������ˮ |
| D������ʱ���ӹ۲�Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��22.30mL | B��22.35mL | C��23.65mL | D��23.70 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������þ�Ż�����ĭ�������� |
| B������Ũ������ͭ��Ӧʵ���ķ�Һ����ˮ�ز���ˮ������ˮ�� |
| C������������Һ�����ڴ�ĥ�ڲ��������Լ�ƿ |
| D����������й¶ʱ���÷���ˮʪ���������棬��Ѹ���뿪�ֳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�____________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________���ٶ����ճ���С�ձ�������Ϊ________(�32.6 g����32.61 g��)��
��ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�____________(����ڡ����ڡ�)�ұߵ����̣���ʹ��ƽƽ�⣬�����еIJ���Ϊ____________���ٶ����ճ���С�ձ�������Ϊ________(�32.6 g����32.61 g��)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ����8.75gʳ�� |
| B����500ml������ƿ����480ml��Һ |
| C����10ml��Ͳ��ȡ6.46ml���� |
| D���ù㷺pH��ֽ���ij��Һ��pHΪ4.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢ� | B���٢� | C���ڢۢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
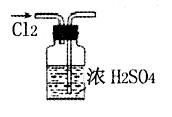
A������Cl2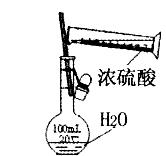 |
B������100ml 0.1mol��L-1������Һ  |
C����ȡ��������ˮ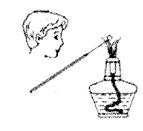 |
| D�������Ԫ�صĴ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com