
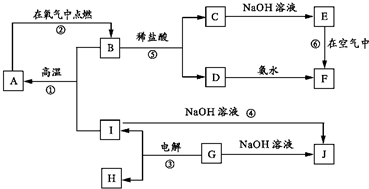
A~I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�F�Ǻ��ɫ���塣
| |||
| | |||
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ���� ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A-I�ֱ��ʾ��ѧ��ѧ�еij������ʣ�����֮����ת����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪G��һ�����������A��B��C��D��E��F���������о�����ͬһ��Ԫ�أ�FΪ���ɫ������
A-I�ֱ��ʾ��ѧ��ѧ�еij������ʣ�����֮����ת����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г���������֪G��һ�����������A��B��C��D��E��F���������о�����ͬһ��Ԫ�أ�FΪ���ɫ������
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����д���пհף�
(1)A��B��C��D��E��F��������������ͬһ��Ԫ����Ԫ�����ڱ���λ��________��
(2)д��C��H���ʵĻ�ѧʽ��C______________________��H_____________________��
(3)д����Ӧ�٢ߵĻ�ѧ����ʽ��
��Ӧ�٣�____________________________________________________________��
��Ӧ�ߣ�_____________________________________________________________��
(4)��Ӧ�����е�������____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���½���³ľ��һ�и����ڶ����¿���ѧ�Ծ� ���ͣ������
A-I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г�������֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ���λ��
��2��д��C��H���ʵĻ�ѧʽ��C �� H
��3��д����Ӧ�١��Ļ�ѧ����ʽ�ۡ͢��ߵ����ӷ���ʽ��
��Ӧ�٣�________________________________
��Ӧ�ޣ�________________________________
��Ӧ�ۣ�________________________________
��Ӧ�ߣ�________________________________
��4����Ӧ�����е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�ϲ������ѧУ������ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
��8�֣� A-I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ�����ַ�Ӧ�������û���г�������֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�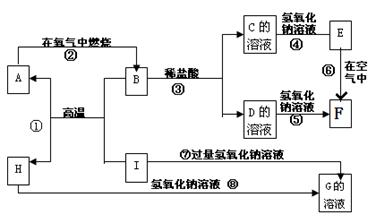
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ���λ��
��2��д��C��H���ʵĻ�ѧʽ��C �� H [��Դ:ѧ����ZXXK]
��3��д����Ӧ�١��Ļ�ѧ����ʽ�ۡ͢��ߵ����ӷ���ʽ��
��Ӧ�٣�________________________________
��Ӧ�ޣ�________________________________
��Ӧ�ۣ�_______________ _________________
_________________
��Ӧ�ߣ�________________________________
��4����Ӧ�����е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ݷ��ʵ����и����������¿���ѧ�Ծ� ���ͣ������
�� 10�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�أ�F�Ǻ��ɫ���塣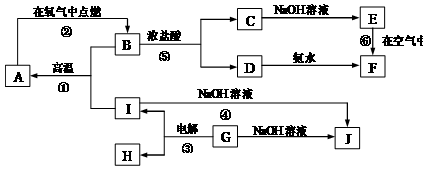
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ���� ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com