
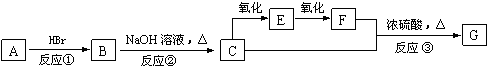
���� ����A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־������A����ϩ���ṹ��ʽΪCH2=CH2��CH2=CH2���廯�ⷢ���ӳɷ�Ӧ����BΪCH3CH2Br��B�ڼ���������ˮ���C�ṹ��ʽΪCH3CH2OH��CH3CH2OH����������EΪCH3CHO��E����������FΪCH3COOH��G��һ����״�й���ζ�����ʣ�CH3CH2OH��CH3COOH����������Ӧ����GΪCH3COOCH2CH3���Դ˽����⣮
��� �⣺����A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־������A����ϩ���ṹ��ʽΪCH2=CH2��CH2=CH2���廯�ⷢ���ӳɷ�Ӧ����BΪCH3CH2Br��B�ڼ���������ˮ���C�ṹ��ʽΪCH3CH2OH��CH3CH2OH����������EΪCH3CHO��E����������FΪCH3COOH��G��һ����״�й���ζ�����ʣ�CH3CH2OH��CH3COOH����������Ӧ����GΪCH3COOCH2CH3��
��1��AΪCH2=CH2��A���ӵĺ˴Ź�������ͼ����1��壬��ѡA��
��2����������ķ�����֪����Ӧ�ڵķ�Ӧ����Ϊ ȡ����Ӧ����ѡA��
��3����EΪCH3CHO��FΪCH3COOH��E��F���������ŵ����Ʒֱ�Ϊȩ�����Ȼ���
�ʴ�Ϊ��ȩ�����Ȼ���
��AΪCH2=CH2��A�Ŀռ乹��Ϊƽ���ͣ�
�ʴ�Ϊ��ƽ���ͣ�
��GΪCH3COOCH2CH3��G��һ��ͬ���칹�����C��E�еĹ����ż������ǻ���ȩ������ṹ��ʽΪCH2��OH��CH2CH2CHO��
�ʴ�Ϊ��CH2��OH��CH2CH2CHO��
�ܷ�Ӧ�۵Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH $?_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH $?_{��}^{Ũ����}$CH3COOC2H5+H2O��
�ݸ�������ķ�����֪��E�Ľṹ��ʽΪCH3CHO��
�ʴ�Ϊ��CH3CHO��
���� ���⿼���л����ƶϣ�Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬�漰ϩ��������ȩ�����ᡢ�����ȴ���֮���ת������ȷ�л����й����ż������ʼ��ɽ���������ճ����л��ﷴӦ���ͣ���Ŀ�ѶȲ���


 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 L H2O ������Ϊ18 g | |
| B�� | 0.5 mol O2�к��еķ�����ԼΪ6.02��1023 | |
| C�� | 0.1 mol/L Na2CO3��Һ�к�Na+�����ʵ���Ϊ0.1 mol | |
| D�� | ���³�ѹ�£�1.7 g NH3���е�������ԼΪ6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH��Һ | B�� | ���� | C�� | Ũ���� | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X��Z����Ԫ�ؿ��γ�X2Z��X2Y2���ֳ������ۻ����� | |
| B�� | ��ҵ�ϳ��õ�����ڵ�W��M�Ȼ���ķ����ֱ���ȡW��M���ֵ��� | |
| C�� | M�������������Y��W��Ԫ������������Ӧˮ������ܷ�Ӧ�����κ�ˮ | |
| D�� | X��W���γ����ӻ�����Ҹ����ӻ�������н�ǿ��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol | B�� | 0.15 mol | C�� | 0.22 mol | D�� | 0.05 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C��M+����C��OH-����C��A-����C��H+�� | B�� | C��M+����C��A-����C��H+����C��OH-�� | ||
| C�� | C��M+����C��A-����C��OH-����C��H+�� | D�� | C��M+��+C��H+����C��A-��+C��OH-�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com