
��1��A���Է����ķ�Ӧ�� ��ѡ����ţ���
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ������� �� ��
��3��B������û��֧������ṹ��ʽ�� ��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�� ��
��4����B��ȡA�Ļ�ѧ����ʽ��
��
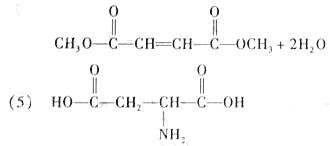
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
![]()
���Ŷ�����Ľṹ��ʽ�� ��
�𰸣�
��1���٢ۢ�
��2��̼̼˫�����Ȼ�
��3��
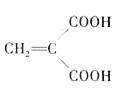
��4��
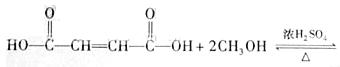
����������A��������������ˮ���B��CH3OH����֪AΪ������BΪ���ᡣ����B�ķ���ʽΪC4H4O4����֪ÿ����B�к�2����COOH��B���ӱ�̼ͬԭ�ӵı��Ͷ�Ԫ����(��HOOCCH2CH2COOH)������Hԭ�ӣ���֪B�г����Ȼ��⣬����C=C����ȷ��B�Dz����Ͷ�Ԫ���ᣬA�Dz������������е�C=C�ܷ����ӳɡ��Ӿۡ������ȷ�Ӧ��B��HClֻ���ܷ����ӳɷ�Ӧ��
HOOC��CH=CH��COOH��HCl����![]()
������NH3����ȡ����Ӧ��
![]() +NH3����
+NH3����![]() �����Ŷ����ᣩ+HCl
�����Ŷ����ᣩ+HCl


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| B |
|
C |
|
���Ŷ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��A���ܷ����ķ�Ӧ��___________(ѡ�����)��
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ�������___________��___________��
��A3��B������û��֧������ṹ��ʽ��_________����B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��_____________��
��4����B��ȡA�Ļ�ѧ����ʽ��______________________��
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
![]()
���Ŷ�����Ľṹ��ʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A��C6H8O4��ΪʳƷ��װ���ڵij��÷�������A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B��C4H4O4���ͼ״���ͨ��״̬��BΪ��ɫ���壬��������������Һ������Ӧ��
��1��B�������������ŵ�������___________________________________________________��
��2��B������û��֧������ṹ��ʽ��_____________________________________________��
��3��A�Ľṹ��ʽ��_____________________________________________________________��
��4�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
B![]() C
C![]() ���Ŷ�����
���Ŷ�����
���Ŷ�����Ľṹ��ʽ��________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A��C6H8O4��ΪʳƷ��װֽ�ij��÷�������A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B��C4H4O4���ͼ״���ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ��
��1��A���Է����ķ�Ӧ��____________��ѡ����ţ���
�� �ӳɷ�Ӧ�� ������Ӧ�� �Ӿ۷�Ӧ�� ������Ӧ
��2��B�������������ŵ�������_____________��_____________��
��3��B������û��֧������ṹ��ʽ��_____________��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��_____________��
��4����B��ȡA�Ļ�ѧ����ʽ��_________________________________________________��
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
![]()
���Ŷ�����Ľṹ��ʽ��________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ�߶���ѧ�����п��Ի�ѧ���� ���ͣ������
��11�֣��л���A��C6H8O4��ΪʳƷ��װֽ�ij��÷�������A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B��C4H4O4���ͼ״���ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ��
��1��A���Է����ķ�Ӧ�� ��ѡ����ţ���
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ������� ��
��3��B������û��֧������ṹ��ʽ�� ��B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�� ��
��4����B��ȡA�Ļ�ѧ����ʽ�� ��
��5�����Ŷ����ᣨC4H7NO4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��

���Ŷ�����Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com