
25 °ś£¨101 k Pa Ī£¨«ŅňŠ”Ž«ŅľÓĶńŌ°»‹“ļ∑Ę…ķ÷–ļÕ∑ī”¶…ķ≥…1molňģ Īňý∑Ň≥ŲĶń»»ŃŅ‘ľő™57.3 kJ/mol£Ľ–ŃÕťĶń»ľ…’»»ő™5518 kJ/mol°£Ō¬Ń–»»ĽĮ—ß∑Ĺ≥Ő Ĺ ť–ī’ż»∑Ķń «£® £©
A£ģCH3COOH(aq)+NaOH(aq)===
CH3COONa(aq)+H2O(l)  H=
H=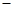 57.3kJ/mol
57.3kJ/mol
 B£ģKOH(aq)+
B£ģKOH(aq)+ H2SO4(aq)=
H2SO4(aq)=  K2SO4(aq)+H2O(l)
K2SO4(aq)+H2O(l)
 H=
H=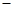 57.3kJ/mol
57.3kJ/mol
C£ģ2C8H18(g)+25O2
(g)=16CO2 (g)+18H2O(1)  H=
H=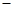 11036
kJ/mol
11036
kJ/mol
 D£ģC8H18(l)+
D£ģC8H18(l)+ 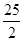 O2 (g)=8CO2 (g)+
9H2O(g)
O2 (g)=8CO2 (g)+
9H2O(g)  H=
H=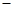 5518 kJ/mol
5518 kJ/mol
 ŐžŐžŌÚ…Ō“ĽĪĺļ√ĺŪŌĶŃ–īūįł
ŐžŐžŌÚ…Ō“ĽĪĺļ√ĺŪŌĶŃ–īūįł –°—ß…ķ10∑÷÷””¶”√Ő‚ŌĶŃ–īūįł
–°—ß…ķ10∑÷÷””¶”√Ő‚ŌĶŃ–īūįł
| ńÍľ∂ | łŖ÷–Ņő≥Ő | ńÍľ∂ | ≥ű÷–Ņő≥Ő |
| łŖ“Ľ | łŖ“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű“Ľ | ≥ű“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ∂Ģ | łŖ∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű∂Ģ | ≥ű∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ»ż | łŖ»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű»ż | ≥ű»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° |
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ2012Ĺžļ”ńŌ °ĹĻ◊ų –łŖ»żĶŕ“Ľīő÷ ŃŅľž≤‚ņŪ◊ŘĽĮ—ß≤Ņ∑÷£®īÝĹ‚őŲ£© Ő‚–Õ£ļŐÓŅ’Ő‚
(15∑÷)÷–—߼Į—ß≥£ľŻ≤Ņ∑÷‘™ňō‘≠◊”ĹŠĻĻľį–‘÷ »ÁŌ¬ĪŪňý ĺ£ļ
| –ÚļŇ | ‘™ňō | ĹŠĻĻľį–‘÷ |
| ĘŔ | A | AĶ•÷ «…ķĽÓ÷–≥£ľŻĹū Ű£¨ňŁ”–ŃĹ÷÷¬»ĽĮőÔ£¨Ōŗ∂‘∑÷◊”÷ ŃŅŌŗ≤Ó35.5 |
| Ęŕ | B | B‘≠◊”K°ĘL°ĘM≤„ĶÁ◊” ż÷ģĪ» «1:4:1 |
| ĘŘ | C | C «ĽÓ∆√∑«Ĺū Ű‘™ňō£¨∆šĶ•÷ ≥£ő¬Ō¬≥ ∆ÝŐ¨ĶęĽĮ—ß–‘÷ ő»∂® |
| Ę‹ | D | DĶ•÷ ĪĽ”Ģő™°į–ŇŌĘłÔ√ŁĶńīŖĽĮľŃ°Ī£¨ «≥£”√ĶńįŽĶľŐŚ≤ńŃŌ[ņī‘ī:Z*xx*k.Com] |
| Ę› | E | Õ®≥£«ťŅŲŌ¬£¨E√Ľ”–’żĽĮļŌľŘ£¨A°ĘC°ĘF∂ľń‹”ŽE–ő≥…∂Ģ÷÷ĽÚ∂Ģ÷÷“‘…ŌĽĮļŌőÔ |
| Ęř | F | F‘ŕ÷‹∆ŕĪŪ÷–Ņ…“‘ŇŇ‘ŕĘŮA◊Ś£¨“≤”–»ňŐŠ≥ŲŇŇ‘ŕĘųA◊Ś |
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ2014Ĺž’„Ĺ≠ °łŖ∂Ģ…Ō—ß∆ŕ∆ŕń©Ņľ ‘ĽĮ—ß ‘ĺŪ£®Ĺ‚őŲįś£© Ő‚–Õ£ļ—°‘ŮŐ‚
25 °ś£¨101 k Pa Ī£¨«ŅňŠ”Ž«ŅľÓĶńŌ°»‹“ļ∑Ę…ķ÷–ļÕ∑ī”¶Ķń÷–ļÕ»»ő™-57.3 kJ/mol£¨–ŃÕťĶń»ľ…’»»ő™-5518 kJ/mol°£Ō¬Ń–»»ĽĮ—ß∑Ĺ≥Ő Ĺ ť–ī’ż»∑Ķń «£® £©
A£ģ2H+(aq)
+ (aq)+
(aq)+ (aq)+2
(aq)+2 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H O(l);
O(l);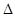 H =
H = 57.3 kJ/mol
57.3 kJ/mol
B£ģKOH(aq)+ H
H SO4(aq) =
SO4(aq) =  K
K SO4(aq)+H
SO4(aq)+H O(l);
O(l);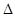 H=
H= 57.3kJ/mol
57.3kJ/mol
C£ģC8H18(l)+
 O
O (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H O(g);
O(g);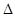 H=
H= 5518 kJ/mol
5518 kJ/mol
D£ģ2C8H18(g)+25O (g)=16CO
(g)=16CO (g)+18H
(g)+18H O(1);
O(1);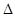 H=
H= 5518 kJ/mol
5518 kJ/mol
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ2013Ĺžį≤Ľ’ °łŖ»żĶŕ“ĽīőÕ≥ŅľĽĮ—ß ‘ĺŪ£®Ĺ‚őŲįś£© Ő‚–Õ£ļ—°‘ŮŐ‚
25 °ś£¨101 k Pa Ī£¨«ŅňŠ”Ž«ŅľÓĶńŌ°»‹“ļ∑Ę…ķ÷–ļÕ∑ī”¶Ķń÷–ļÕ»»ő™57.3 kJ/mol£¨–ŃÕťĶń»ľ…’»»ő™5518 kJ/mol°£Ō¬Ń–»»ĽĮ—ß∑Ĺ≥Ő Ĺ ť–ī’ż»∑Ķń « ( )
A£ģ2H+(aq)+SO42£≠(aq)+Ba2£ę(aq)+2OH-(aq)=BaSO4(s)+2H2O(1)
°ųH= 57.3 kJ/mol
57.3 kJ/mol
B£ģKOH(aq)+ H2 SO4(aq)=
H2 SO4(aq)= K2SO4(aq)+H2O(l) °ųH=
K2SO4(aq)+H2O(l) °ųH= 57.3kJ/mol
57.3kJ/mol
C£ģC8H18(I)+ O2(g)=8CO2(g)+9H2O °ųH=
O2(g)=8CO2(g)+9H2O °ųH= 5518 kJ/mol
5518 kJ/mol
D£ģ2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)
°ųH= 5518 kJ/mol
5518 kJ/mol
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ2013ĹžĻ„∂ę °łŖ∂Ģ…Ō—ß∆ŕ∆ŕ÷–Ņľ ‘ĽĮ—ß ‘ĺŪ Ő‚–Õ£ļ—°‘ŮŐ‚
25°ś£¨101 k Pa Ī£¨«ŅňŠ”Ž«ŅľÓĶńŌ°»‹“ļ∑Ę…ķ÷–ļÕ∑ī”¶Ķń÷–ļÕ»»ő™57.3 kJ/mol£¨–ŃÕťĶń»ľ…’»»ő™5518 kJ/mol°£Ō¬Ń–»»ĽĮ—ß∑Ĺ≥Ő Ĺ ť–ī’ż»∑Ķń «£® £©
A£ģ2H+(aq) +SO42£≠(aq)+Ba2+(aq)+2OH£≠(aq)=BaSO4(s)+2H2O(1)£Ľ¶§H=£≠57.3 kJ/mol
B£ģKOH(aq)+ H2 SO4(aq)=K2SO4(aq)+H2O(l)£Ľ¶§H=£≠57.3kJ/mol
C£ģ2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)£Ľ¶§H=£≠5518 kJ/mol
D£ģC8H18(l)+ 25/2 O2(g)=8CO2(g)+ 9H2O(1)£Ľ¶§H=£≠5518 kJ/mol
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ2009łŖŅľ’śŐ‚Ľ„Īŗ-—ű◊Ś‘™ňō£¨Ľ∑ĺ≥Ī£Ľ§ Ő‚–Õ£ļ—°‘ŮŐ‚
25 °ś£¨101 k Pa Ī£¨«ŅňŠ”Ž«ŅľÓĶńŌ°»‹“ļ∑Ę…ķ÷–ļÕ∑ī”¶Ķń÷–ļÕ»»ő™57.3 kJ/mol£¨–ŃÕťĶń»ľ…’»»ő™5518 kJ/mol°£Ō¬Ń–»»ĽĮ—ß∑Ĺ≥Ő Ĺ ť–ī’ż»∑Ķń «
A.2H+(aq) + (aq)+
(aq)+ (aq)+2OH
(aq)+2OH (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H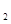 O(1);
O(1); H=
H= 57.3
kJ/mol
57.3
kJ/mol
 B.KOH(aq)+
B.KOH(aq)+ H
H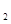 SO4(aq)=
SO4(aq)=  K
K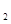 SO4(aq)+H
SO4(aq)+H O(I);
O(I);  H=
H= 57.3kJ/mol
57.3kJ/mol
C.C8H18(I)+  O
O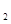 (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H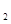 O;
O;  H=
H= 5518 kJ/mol
5518 kJ/mol
 D.2C8H18(g)+25O
D.2C8H18(g)+25O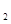 (g)=16CO
(g)=16CO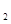 (g)+18H
(g)+18H O(1);
O(1);  H=
H= 5518 kJ/mol
5518 kJ/mol
≤ťŅīīūįłļÕĹ‚őŲ>>
Ļķľ —ß–£”Ň—° - Ń∑Ōį≤ŠŃ–ĪŪ - ‘Ő‚Ń–ĪŪ
ļĢĪĪ °Ľ•Ń™ÕÝő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®∆ĹŐ® | ÕÝ…Ō”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | ĶÁ–Ň’©∆≠ĺŔĪ®◊®«Ý | …śņķ ∑–ťőř÷ų“Ś”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | …ś∆ů«÷»®ĺŔĪ®◊®«Ý
ő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®ĶÁĽį£ļ027-86699610 ĺŔĪ®” Ōš£ļ58377363@163.com