
| A���٢ܢݢ� | B���ۢܢ� |
| C���٢ڢ� | D���ڢۢݢ� |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��1 | B��1��3 |
| C��2��3 | D��3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����58.5g NaCl����1Lˮ�пɵ�1mol?L-1��NaCl��Һ |
| B������״����22.4L HCl����1Lˮ�пɵ�1mol?L-1���� |
| C����25.0g��������ˮ�����100mL��Һ��������Һ�����ʵ���Ũ��Ϊ1mol?L-1 |
| D����78g Na2O2����ˮ�õ�1L��Һ�������ʵ���Ũ��Ϊ1mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��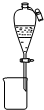 �������еĵ�;ƾ� |
B��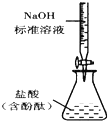 �ⶨ�����Ũ�� |
C��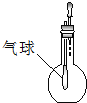 ��֤HCl���ܽ��� |
D��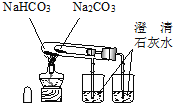 �Ƚ�Na2CO3��NaHCO3�����ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ӧ��Ũ�ȣ�������λ����ڻ���ӵİٷ������Ӷ�ʹ��Ӧ�������� |
| B��������μӵĻ�ѧ��Ӧ��������ѹǿ������С��Ӧ������������������ӻ���ӵİٷ������Ӷ�ʹ��Ӧ�������� |
| C�������¶���ʹ��ѧ��Ӧ�����������Ҫԭ���������˷�Ӧ���л���ӵİٷ��� |
| D������������λ����ڻ���ӵİٷ������Ӷ�����Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Mgʧ������CO2���Naʧ��Ҳ������CO2��� |
| B��Al��Sֱ�ӻ��Ͽ��Եõ�Al2S3��Fe��Sֱ�ӻ���Ҳ���Եõ�Fe2S3 |
| C����SO2ͨ��BaCl2��Һ�ް�ɫ�������ɣ�SO2ͨ��Ba��NO3��2��ҺҲ�ް�ɫ�������� |
| D����ҵ�ϵ������MgCl2��ȡ����þ��Ҳ�õ������AlCl3�ķ�����ȡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaCl�к�������Na2SO4�����ᱵ�� |
| B��FeSO4 �к�������CuSO4�����ۣ� |
| C��CO2�к�������HCl���壨����������Һ�� |
| D��CO2�к���������CO�����ȵ�����ͭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʹ�÷�Һ©��ǰ���ü��������Ƿ�©ˮ |
| B����Һ����ʱ���²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| C��ѡ��CCl4��ƾ���Ϊ��ȡ���ӵ�ˮ����ȡ�� |
| D��Ϊ���ٹ������ʵ��ܽ�ɲ��÷��顢������ȷ��� |
| E������NaCl��Һʱ���ò��������ֹҺ��ֲ���������ɽ� |
| F������H2SO4��Һʱ��������Ͳ�м�һ�����ˮ������������ŨH2SO4����ʱ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.075 | B��0.8 |
| C��0.75 | D��0.08 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com