���𰸡�
��������1������ԭ���������������ԭ����д�����Ų�ʽ�������������ʺ͵���������غ㶨����д���ӷ���ʽ��
��2������������=������+�����������������������ݾ�������ʺͳɼ���ʽ�жϾ�������ͣ�
��3����Ԫ�����ڱ���ֻ�еڢ�A��±��ԭ�ӵ��⻯���ˮ��Һ�ž������ԣ�����Ӱ����Ӿ�����۷е�ߵ͵������ж��۷е�ߵͣ���һ���ƶ����ʣ�
��4����ҩƤ�ijɷֺ����ʵ������ƶϣ�
��5�����������غ㶨�ɺͻ�ѧ����ʽ���м��㣮
����⣺��1��Al�����ڱ���λ�ڵ������ڵڢ�A�壬Alԭ�Ӻ��13�����ӣ������������ԭ�����������������͵�1s��������������������������ϸߵĹ��������Ų�ʽΪ1S
22S
22P
63S
23P
1��Al��ǿ����Һ��Ӧ����AlO
2-��H
2�����ӷ���ʽΪ2Al+2OH
-+2H
2O=2AlO
2-+3H
2�����ʴ�Ϊ��1S
22S
22P
63S
23P
1�� 2Al+2OH
-+2H
2O=2AlO
2-+3H
2����
��2����ԭ�ӷ��ϵı���ʽ�����ϽDZ�ʾ�����������½DZ�ʾ������������������=������+�������ɼ����
30Si��ԭ�ӵ�������Ϊ��30-14=16��SiO
2�۵�ߡ�Ӳ�ȴ�Ϊԭ�Ӿ��壬�ʴ�Ϊ��16�� ԭ�Ӿ��壻
��3����Ԫ�����ڱ���ֻ�еڢ�A��±��ԭ�ӵ��⻯���ˮ��Һ�ž������ԣ���ΪAl
3+��Y
n-�ĵ�������ͬ������Y��FԪ�أ�±��Ԫ���γɵ��⻯������ڷ��Ӿ��壬��е�����Ӽ�����������������ߣ�������HF�����д�����������HF�ķе���ߣ����Էе���͵���HCl���ʴ�Ϊ��HCl��
��4����ҩƤ�ijɷִ���ʯ��ˮ�ࡢ������֪���ڸ�����ֻ�д���ʯ�ŷֽ����CO
2���������ֻ����CO
2���壬�����к���2���ļ���ӦΪSP�ӻ����ʴ�Ϊ��CO
2��SP�ӻ���
��5��������ֻ��SiO
2�������Ӧ�����11.0g��SiO
2��������Fe
2O
3��Al
2O
3��������ֱ�����FeCl
3��AlCl
3������Һ�м������NaOH��ҺʱAlCl
3����NaAlO
2��FeCl
3����Fe��OH��
3����������21.4g������Fe��OH��
3�������������ʵ���Ϊ
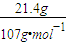
=0.2 mol������ԭ���غ�֪Fe
2O
3�����ʵ���Ϊ0.1mol��������Ϊ0.1mol×160g?mol
-1=16.0g��������Al
2O
3����������Ϊ ��36.0-11.0-16.0��/36×100%=25%���ʴ�Ϊ��25%��
���������⿼��ԭ�ӵĽṹ���������͡�����ʽ����д�Լ��йؼ��㣬��Ŀ��Ϊ�ۺϣ�������ע������жϾ��������Լ������۷е�ߵ͵ķ�����

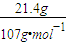 =0.2 mol������ԭ���غ�֪Fe2O3�����ʵ���Ϊ0.1mol��������Ϊ0.1mol×160g?mol-1=16.0g��������Al2O3����������Ϊ ��36.0-11.0-16.0��/36×100%=25%���ʴ�Ϊ��25%��
=0.2 mol������ԭ���غ�֪Fe2O3�����ʵ���Ϊ0.1mol��������Ϊ0.1mol×160g?mol-1=16.0g��������Al2O3����������Ϊ ��36.0-11.0-16.0��/36×100%=25%���ʴ�Ϊ��25%��