
(10·ЦЈ©УРAЎўBЎўCЎўDЛДЦЦАлЧУ»ЇєПОпЈ¬ЧйіЙЛьГЗµДАлЧУ·Ц±рОЄЈє
СфАлЧУЈєNaЈ«ЎўAl3Ј«ЎўNH4+Ј» ТхАлЧУЈєOHЈЎўNO3ЈЎўCO32ЈЎўHSO4Ј
ОЄјш±рЛДЦЦ»ЇєПОпЈ¬ДіС§Йъ·Ц±рИЎЙЩБї№ММеЕдіЙИЬТєЈ¬±аєЕОЄAЎўBЎўCЎўDЅшРРКµСйЎЈКµСй№эіМєНјЗВјИзПВНјЛщКѕЈЁОЮ№ШОпЦКТСВФИҐЈ©
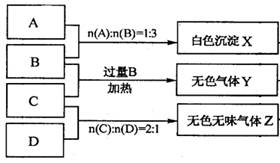
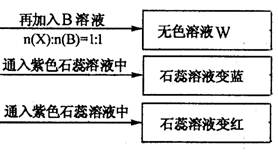
(1) YЎўZµД»ЇС§КЅ·Ц±рОЄЈєY Ј»Z
(2)РґіцЦё¶Ё·ґУ¦µДАлЧУ·ЅіМКЅЈє
ўЩјУИИМхјюПВЈ¬CУл№эБїB·ґУ¦Јє
ўЪDИЬТєПФИхјоРФµДФТтКЗ(УГАлЧУ·ЅіМКЅ±нКѕ)
(3)µИОпЦКµДБїЕЁ¶ИµДAЎўBЎўCЎўDИЬТєpHУЙґуµЅРЎµДЛіРтКЗ(УГ»ЇС§КЅ±нКѕ)
(4)ИфBЎўCµДПЎИЬТє»мєПєу(І»јУИИ)ИЬТєіКЦРРФЈ¬ФтИЬТєЦРАлЧУЕЁ¶ИґУґуµЅРЎµДЛіРтКЗЈє
(1)NH3(1·Ц) CO2(1·Ц)
(2)ўЩNH4++H++2OH-ЈЅNH3Ўь+2H2O(2·Ц)
ўЪCO32-+H2O HCO3-+OH-
(2·Ц)
HCO3-+OH-
(2·Ц)
(3) NaOH>Na2CO3>Al(NO3)3>NH4HSO4(2·Ц)
(4) c(Na+)>c(SO42-)>c(NH4+)>c(H+)=c(OH-)(2·Ц)
ЎѕЅвОцЎїЈЁ1Ј©ДЬК№ЧПЙ«µДКЇИпКФТєПФА¶Й«µДКЗ°±ЖшЈ¬ДЬК№ЧПЙ«КЇИпКФТєПФємЙ«µДКЗЛбРФЖшМеЈ¬ЛщТФёщѕЭМвТвїЙЦЄYКЗ°±ЖшЈ¬ZКЗCO2ЎЈ
ЈЁ2Ј©°ЧЙ«іБµнXДЬИЬУЪBЦРЈ¬ТтґЛіБµнЗвСх»ЇВБЈ¬ЛщТФёщѕЭ·ґУ¦µДОпЦКµДБїЦ®±ИїЙЦЄЈ¬AКЗПхЛбВБЈ¬BКЗЗвСх»ЇДЖЎЈCКЗБтЛбЗвп§Ј¬DКЗМјЛбДЖЎЈ
ўЩјУИИМхјюПВЈ¬CУл№эБїB·ґУ¦µД·ЅіМКЅОЄNH4++H++2OH-ЈЅNH3Ўь+2H2OЎЈ
ўЪМјЛбДЖКЗЗїјоИхЛбСОЈ¬CO32ЈЛ®ЅвПФјоРФЈ¬·ЅіМКЅОЄCO32-+H2O HCO3-+OH-ЎЈ
HCO3-+OH-ЎЈ
ЈЁ3Ј©ЗвСх»ЇДЖКЗЗїјоЈ¬јоРФЧоЗїЎЈМјЛбДЖЛ®ЅвПФјоРФЈ¬ПхЛбВБЛ®ЅвПФЛбРФЈ¬БтЛбЗв淋зАліцЗвАлЧУПФЛбРФЈ¬ЛщТФµИОпЦКµДБїЕЁ¶ИµДAЎўBЎўCЎўDИЬТєpHУЙґуµЅРЎµДЛіРтКЗNaOH>Na2CO3>Al(NO3)3>NH4HSO4ЎЈ
ЈЁ4Ј©BЎўCµДПЎИЬТє»мєПєу(І»јУИИ)ИЬТєіКЦРРФЈ¬ФтИЬТєЦРє¬УРµДИЬЦККЗБтЛбДЖЎўБтЛбп§єН°±Л®Ј¬ЛщТФИЬТєЦРАлЧУЕЁ¶ИґУґуµЅРЎµДЛіРтКЗ c(Na+)>c(SO42-)>c(NH4+)>c(H+)=c(OH-)ЎЈ


 МЖУЎОД»ЇїОК±ІвЖАПµБРґр°ё
МЖУЎОД»ЇїОК±ІвЖАПµБРґр°ё µјС§УлІвКФПµБРґр°ё
µјС§УлІвКФПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
ЈЁ±ѕМв№І10·ЦЈ©УРAЎўBЎўCЎўDЎўEЎўFБщЦЦФЄЛШЎЈ
ўЩAЎўBЎўCКЗЅрКфФЄЛШЈ¬О»УЪН¬Т»ЦЬЖЪЈ¬ФЧУєЛНв¶јУР3ёцµзЧУІгЈ¬AµДФЧУ°лѕ¶ФЪЛщКфЦЬЖЪЦРЧоґуЈ¬ЗТФЧУ°лѕ¶A>B>CЎЈ
ўЪDЎўEКЗ·ЗЅрКфФЄЛШЈ¬ЛьГЗёъЗв»ЇєПїЙЙъіЙЖшМ¬Зв»ЇОпHDєНHEЈ¬ФЪКТОВК±Ј¬DµДµҐЦККЗТєМеЈ¬EµДµҐЦККЗ№ММеЎЈ
ўЫFµДµҐЦКФЪіЈОВПВКЗЖшМеЈ¬РФЦКєЬОИ¶ЁЈ¬КЗіэЗвНвЧоЗбµДЖшМеЎЈ
Зл»ШґрЈє
ЈЁ1Ј©BО»УЪЦЬЖЪ±нЦРµЪ________ЦЬЖЪ______ЧеЈ¬CµДФЧУЅб№№КѕТвНјКЗ________ЎЈ
ЈЁ2Ј©FµҐЦКµД»ЇС§КЅКЗ________ЎЈ
ЈЁ3Ј©ФЪЙПКцБщЦЦФЄЛШЦРЈ¬ЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпјоРФЧоЗїµДОпЦККЗ_____Ј¬ЛбРФЧоЗїµДОпЦККЗ________Ј¬ЖшМ¬Зв»ЇОпЧоОИ¶ЁµДКЗ________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2009ЎЄ2010С§Дк№гЦЭКРЖЯЗшБЄїјёЯТ»»ЇС§ПВС§ЖЪЖЪД©јаІв МвРНЈєМоїХМв
ЈЁ±ѕМв№І10·ЦЈ©УРAЎўBЎўCЎўDЎўEЎўFБщЦЦФЄЛШЎЈ
ўЩAЎўBЎўCКЗЅрКфФЄЛШЈ¬О»УЪН¬Т»ЦЬЖЪЈ¬ФЧУєЛНв¶јУР3ёцµзЧУІгЈ¬AµДФЧУ°лѕ¶ФЪЛщКфЦЬЖЪЦРЧоґуЈ¬ЗТФЧУ°лѕ¶A>B>CЎЈ
ўЪDЎўEКЗ·ЗЅрКфФЄЛШЈ¬ЛьГЗёъЗв»ЇєПїЙЙъіЙЖшМ¬Зв»ЇОпHDєНHEЈ¬ФЪКТОВК±Ј¬DµДµҐЦККЗТєМеЈ¬EµДµҐЦККЗ№ММеЎЈ
ўЫFµДµҐЦКФЪіЈОВПВКЗЖшМеЈ¬РФЦКєЬОИ¶ЁЈ¬КЗіэЗвНвЧоЗбµДЖшМеЎЈ
Зл»ШґрЈє
ЈЁ1Ј©BО»УЪЦЬЖЪ±нЦРµЪ________ЦЬЖЪ______ЧеЈ¬CµДФЧУЅб№№КѕТвНјКЗ________ЎЈ
ЈЁ2Ј©FµҐЦКµД»ЇС§КЅКЗ________ЎЈ
ЈЁ3Ј©ФЪЙПКцБщЦЦФЄЛШЦРЈ¬ЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпјоРФЧоЗїµДОпЦККЗ_____Ј¬ЛбРФЧоЗїµДОпЦККЗ________Ј¬ЖшМ¬Зв»ЇОпЧоОИ¶ЁµДКЗ________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2011-2012Дкєю±±ТЛІэЅр¶«·ЅС§РЈёЯ¶юЙПС§ЖЪЖЪД©їјКФ»ЇС§КФѕнЈЁґшЅвОцЈ© МвРНЈєМоїХМв
(10·ЦЈ©УРAЎўBЎўCЎўDЛДЦЦАлЧУ»ЇєПОпЈ¬ЧйіЙЛьГЗµДАлЧУ·Ц±рОЄЈє
СфАлЧУЈєNaЈ«ЎўAl3Ј«ЎўNH4+Ј» ТхАлЧУЈєOHЈЎўNO3ЈЎўCO32ЈЎўHSO4Ј
ОЄјш±рЛДЦЦ»ЇєПОпЈ¬ДіС§Йъ·Ц±рИЎЙЩБї№ММеЕдіЙИЬТєЈ¬±аєЕОЄAЎўBЎўCЎўDЅшРРКµСйЎЈКµСй№эіМєНјЗВјИзПВНјЛщКѕЈЁОЮ№ШОпЦКТСВФИҐЈ©
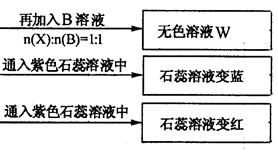
(1) YЎўZµД»ЇС§КЅ·Ц±рОЄЈєY Ј»Z
(2)РґіцЦё¶Ё·ґУ¦µДАлЧУ·ЅіМКЅЈє
ўЩјУИИМхјюПВЈ¬CУл№эБїB·ґУ¦Јє
ўЪDИЬТєПФИхјоРФµДФТтКЗ(УГАлЧУ·ЅіМКЅ±нКѕ)
(3)µИОпЦКµДБїЕЁ¶ИµДAЎўBЎўCЎўDИЬТєpHУЙґуµЅРЎµДЛіРтКЗ(УГ»ЇС§КЅ±нКѕ)
(4)ИфBЎўCµДПЎИЬТє»мєПєу(І»јУИИ)ИЬТєіКЦРРФЈ¬ФтИЬТєЦРАлЧУЕЁ¶ИґУґуµЅРЎµДЛіРтКЗЈє
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2009-2010С§Дк№гЦЭКРЖЯЗшБЄїјёЯТ»»ЇС§ПВС§ЖЪЖЪД©јаІв МвРНЈєМоїХМв
ЈЁ±ѕМв№І10·ЦЈ©УРAЎўBЎўCЎўDЎўEЎўFБщЦЦФЄЛШЎЈ
ўЩAЎўBЎўCКЗЅрКфФЄЛШЈ¬О»УЪН¬Т»ЦЬЖЪЈ¬ФЧУєЛНв¶јУР3ёцµзЧУІгЈ¬AµДФЧУ°лѕ¶ФЪЛщКфЦЬЖЪЦРЧоґуЈ¬ЗТФЧУ°лѕ¶A>B>CЎЈ
ўЪDЎўEКЗ·ЗЅрКфФЄЛШЈ¬ЛьГЗёъЗв»ЇєПїЙЙъіЙЖшМ¬Зв»ЇОпHDєНHEЈ¬ФЪКТОВК±Ј¬DµДµҐЦККЗТєМеЈ¬EµДµҐЦККЗ№ММеЎЈ
ўЫFµДµҐЦКФЪіЈОВПВКЗЖшМеЈ¬РФЦКєЬОИ¶ЁЈ¬КЗіэЗвНвЧоЗбµДЖшМеЎЈ
Зл»ШґрЈє
ЈЁ1Ј©BО»УЪЦЬЖЪ±нЦРµЪ________ЦЬЖЪ______ЧеЈ¬CµДФЧУЅб№№КѕТвНјКЗ________ЎЈ
ЈЁ2Ј©FµҐЦКµД»ЇС§КЅКЗ________ЎЈ
ЈЁ3Ј©ФЪЙПКцБщЦЦФЄЛШЦРЈ¬ЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпјоРФЧоЗїµДОпЦККЗ_____Ј¬ЛбРФЧоЗїµДОпЦККЗ________Ј¬ЖшМ¬Зв»ЇОпЧоОИ¶ЁµДКЗ________ЎЈ
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com