
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��1��Ar
��2��HClO4��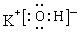
��3��Al��Al2O3+2OH-��2AlO2-+H2O
��4��4NH3��5O2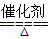 4NO��6H2O��4NH3��3O2
4NO��6H2O��4NH3��3O2 2N2��6H2O
2N2��6H2O
��5��3NO2��H2O=2HNO3��NO
��6��O��C��O���ɱ������ӣ��˹�����
�����������������Ԫ�������ڱ��еķֲ���������֪����C������N������O������Na������Al������S������Cl������Ar������K����
��1������ЩԪ���У���ѧ�������ȶ�����ϡ������Ar��
��2������Ԫ�������ɣ�ͬ����Ԫ�ص�ԭ�ӣ�����������������Ӧˮ�������������ǿ������������ͬ����Ԫ�ص�ԭ�ӣ����µ�������������Ӧˮ�����������������������ǿ������֪������ǿ�ĺ������Ǹ����ᣬ������ǿ����KOH���������������ӻ���������ʽ�ɱ�ʾΪ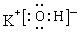 ��
��
��3�����������������������������������������������Ԫ����Al���������ܺ�ǿ�Ӧ�����κ�ˮ�����ӷ���ʽΪAl2O3+2OH-��2AlO2-+H2O��
��4�����⻯���ǰ�����۵ĵ���������һ�������·�Ӧ�Ļ�ѧ����ʽΪ��4NH3��5O2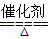 4NO��6H2O��4NH3��3O2
4NO��6H2O��4NH3��3O2 2N2��6H2O��
2N2��6H2O��
��5������ɫ������NO2��NO2����ˮ����NO�����ᣬ��������ˮ���ռ�����Ӧ�Ļ�ѧ����ʽΪ3NO2��H2O=2HNO3��NO
��6��̼�������ɵĻ�������CO2���ṹʽΪO��C��O��CO2�ڹ�̬ʱ�׳Ƹɱ����γɵľ����Ƿ��Ӿ��壬���������˹����ꡣ
���㣺����Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ��


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�A��B��C��D��E��FΪԭ��������������Ķ�����Ԫ�أ�B��C������ͬ���ڣ�A��Dͬ���塣Cԭ�������������Ǻ�����Ӳ�����3����A��C���γ����ֻ�������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1����Ϊ�������ܼ���E�ǵؿ��к������Ľ���Ԫ�أ�FԪ��Ϊͬ���ڵ縺������Ԫ�ء�D��F���γɻ��������E��F���γɻ����ﶡ��GΪ��������δ�ɶԵ���������Ԫ�ء���ش��������⣺
��1��д��G��̬ԭ�ӵļ۵����Ų�ʽ ��
��2�� B��C�Ƚϣ���һ�����ܽϴ���� ����Ԫ�ط��ţ�����ԭ��Ϊ ��
��3���ס��������ӵ�����ԭ�ӵ��ӻ������Ƿ���ͬ ����ͬ������ͬ����
��4����֪�����ﶡ�۵�190�棬�е�183�档���Ͷ��Ƚϣ��۵�ϸߵ��� ���ѧʽ����
��5�����������G3+��ס�Ԫ��F���ɣ���֪����������λ��Ϊ6���ں���0.1mol�����Һ�м���AgNO3��Һ�������������ˡ�ϴ�ӡ�����õ�28.7g��ɫ������������Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣��������ʹ���������������õ���Ҫ�о�����
��1��������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB��NH)3ͨ�����·�Ӧ�Ƶã�3CH4��2(HB��NH)3��6H2O��3CO2��6H3BNH3����ش�
��H3BNH3���Ƿ������λ�� ����ǡ�����B��C��N��O��һ�������ɴ�С��˳��Ϊ ��CH4��H2O��CO2�����Ӱ��ռ����ɴ�С��˳������Ϊ ��
����(HB��NH)3��Ϊ�ȵ�����ķ���Ϊ �������ʽ��
���˹����Ժϳ����һϵ���⻯��������������������ƣ��ʳ�֮Ϊ���顣��ҵ�Ͽɲ���LiAlH4��BCl3��һ���������Ʊ�������B2H6���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�����������У�����������״����״���Ǽ�״�ȶ��ֽṹ��ʽ��ͼ��Ϊһ��������״�ṹ�Ķ���������仯ѧʽΪ ��ͼ��Ϊ��ɰ�����������ӣ�������ԭ�Ӳ�ȡ���ӻ���ʽΪ ��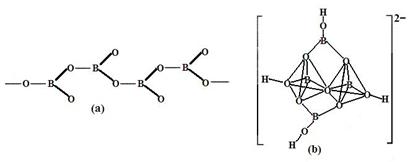
��2��һ��ͭ�Ͻ���д����
��Cu2+�ļ۲�����Ų�ʽΪ ��
��ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ����ԭ���� ��
��ͭ�ĵ����а�ABCABC������ʽ�ѻ�����ͭԭ�Ӱ뾶Ϊa pm����þ�����ܶ�Ϊ g/cm3������٤������ֵΪNA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣�����ѧ�������ʽṹ�����ʡ�������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2 np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӡ�
��1��CԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽ ����AԪ��Ϊ�ǽ���Ԫ�أ�A��C�γɵĻ������еĹ��ۼ����� ����� ����
����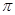 ������
������
��2����n=2ʱ��B�������̬�⻯��ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ��BC2���� ���ӣ�����ԡ��Ǽ��ԡ�������n=3ʱ��B��C�γɵľ�������____ ���壻
��3����AԪ�ص�ԭ�����������Ų�Ϊ2s1��BԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ţ���
��4����ͼΪCԪ�����Ѹ�Ԫ���γɵ�ij����ṹ�е���С�ظ���Ԫ���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ������� �����þ���Ļ�ѧʽΪ ��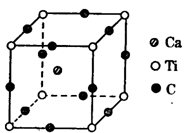
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ�����4����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���壮
��1��D�����ڱ��е�λ�� ��B��ԭ�Ӻ�������Ų�ʾ��ͼ ��
��2��EԪ���γ�����������Ӧˮ����Ļ�ѧʽΪ ��
��3��Ԫ��C��D��E�γɵ�ԭ�Ӱ뾶��С��ϵ�� ����Ԫ�ط��ű�ʾ����
��4��C��D���γɻ�����D2C2��D2C2���еĻ�ѧ���� ��
��5��A��C����Ԫ���γɵ�ԭ�Ӹ���֮��Ϊ1:1�Ļ��������ʽ ��
��6��B���⻯����B������������ˮ���ﷴӦ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ��ѧԪ�ء�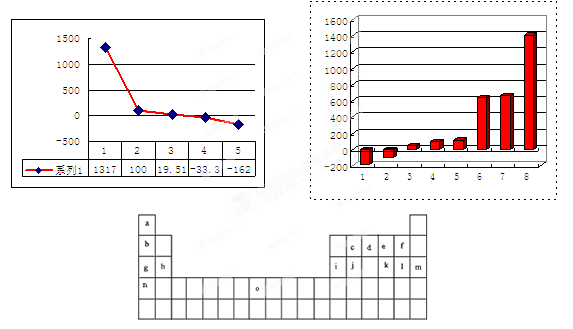
�Իش���������
��1��Ԫ�ء�O�������ڱ��е�λ���� ��
��2��������c���ļ����Ų�ʽ ��
��3����������8��Ԫ�ذ������۵��С˳�������ͼ���ϣ��������С�1������ ������ĸ����
��4��b��c��d��e��f���⻯��ķе㣨�棩ֱ������ͼ���ң����С�5���⻯��Ļ�ѧʽΪ�� �����С�1���⻯��ĵ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��V��W��X��Y��Z����Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������ֻ��X��Z�ǽ���Ԫ�أ�V��ZԪ��ԭ������㶼ֻ��һ�����ӣ�W��YԪ��ԭ�ӵ�������������ͬ����WԪ��ԭ��L���������K���������3����XԪ��ԭ�ӵ�������������YԪ��ԭ��������������һ�룮�ɴ���֪��
V�� ��W�� ��X�� ��Y�� ��Z�� ����Ԫ�ط��ţ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A���л������Σ�B��C��D�dz�������� A��B��C��D��ɫ��Ӧ�ʻ�ɫ��ˮ��Һ���ʼ��ԣ�����B�ļ�����ǿ��X��Y��������������������塢����ϢϢ��أ����ǵľ���������ͬ��A��B�����ʵ�����Ӧ����D��һ�����嵥�ʣ�C���ȷֽ�õ�Y��D��X��B��C��Ӧ����D��X��E������Ԫ����ɣ�ʽ��Ϊ83����EͶ��X�еõ�B������Z��Z�ڱ�״���µ��ܶ�Ϊ0.76g��L-1��
��1��A�Ļ�ѧʽ�� ��Y�ĵ���ʽ�� ��
��2��X�ķе��ͬ����ͬ��������Ҫ�ߣ�ԭ���� ��
��3��д��E���������ᷴӦ�Ļ�ѧ����ʽ
��4��д����D�ı�����Һ�в���ͨY����C�����ӷ���ʽ ��
��5��A��һ����ҪӦ���Ǹ���2A ��P +H2���õ�P��P��Һ�е�������ͨ����CaCl2ʹ֮������������ȫ����ʱ����Һ��Ca2+�����ʵ���Ũ������Ϊ ��
������Ksp=2.3��10-9������Һ������Ũ�ȡ�10-5mol��L-1��������Ϊ��ȫ������
��6��ʵ���ҳ���P������HCl��Ӧ���õ��л�����Ũ���������¹��ȷֽ���ij��ԭ�����壬���ʵ��֤���ֽ�����л�ԭ������Ĵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)ͼ1ΪԪ��X��ǰ�弶�����ܵ���ֵʾ��ͼ����֪X��ԭ������<20����д��X��̬ԭ�ӵĺ�������Ų�ʽ ��
(2)A��B��C��D��E��F��G��H���ֶ�����Ԫ�أ��䵥�ʵķе���ͼ2��ʾ��
��ش�
������Ԫ���У�ijЩԪ�صij����������γɵľ���Ϊ���Ӿ��壬��Щ���ʷ����мȺ��ЦҼ��ֺ��Цм����� (�ѧʽ)��
����֪D��F��G����Ԫ�ص����Ӿ��и�E��ͬ�ĵ��Ӳ�ṹ����B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ(�����Ԫ�ط��ű�ʾ) ��
����֪H�ĵ縺��Ϊ1.5������Ԫ�صĵ縺��Ϊ3.0�������γɵĻ����K��ˮ�⣬�����������ݴ��Ʋ�û�����Ļ�ѧ������Ϊ ��
��ԭ��������AС1��Ԫ����DԪ���γɵĻ�����Ŀռ乹��Ϊ ������ԭ�ӵ��ӻ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com