
ij��Ȼ��Ļ�ѧ��ɿ���ΪaNa2CO3��bNaHCO3��cH2O (a��b��cΪ������)��Ϊȷ������ɣ���ѧ��ȤС���ͬѧ����������ʵ�飺
(1)���Է�����
��ȡ������Ȼ����Ʒ�����Թ��У��þƾ��Ƽ��ȣ����Թܿ���Һ�����ɣ���Һ����ʹ��ˮ����ͭ�������ܷ�˵����Ʒ�к��ᾧˮ���Լ������ɡ�_________________________________��
���������һ����ʵ�鷽����ȷ����Ʒ�к���![]() ��
��
______________________________________________________________________________��
(2)������������С��ͬѧ�������ͼ��ʾװ�ã��ⶨ��Ȼ��Ļ�ѧ��ɡ�
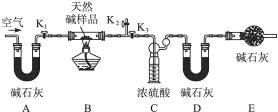
ʵ�鲽�裺
�ٰ���ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����____________________��
A����ʯ�ҵ�������_______________��E����ʯ�ҵ�������__________________________��
�ڳ�ȡ��Ȼ����Ʒ7.3 g�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿ������Ϊ87.6 g��װ��ʯ�ҵ�U�ι�D������Ϊ74.7 g��
�۴���K1��K2���ر�K3������������������ӡ�
�ܹرջ���K1��K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���K1������������������ӣ�Ȼ��Ƶ�װŨ�����ϴ��ƿ����Ϊ88.5 g��װ��ʯ�ҵ�U�ι�D������Ϊ75.8 g���ò����л���������������ӵ�Ŀ����_____________�������Ƶ�������Ȼ��Ļ�ѧʽΪ_____________��
(1)���Է�����
�ٲ��ܣ���ΪNaHCO3���ȷֽ�Ҳ�ܲ���ˮ����
��ȡ������Ʒ����ˮ���μ�CaCl2����BaCl2����Һ���а�ɫ�������ɣ�˵����CO2-3
(2)����������
�ټ��װ�õ������� ��ȥ�����е�CO2��H2O��g��
��ֹ������CO2��H2O(g)����Dװ�õ��²�����ȷ
�ݽ�װ�������ɵ�CO2��H2O��g��ȫ������C��Dװ���б����� Na2CO3��2NaHCO3��H2O
���⿼��Na2CO3��NaHCO3�й����ʡ���������ӵļ��飬�ۺ��Խ�ǿ��(1)����2NaHCO3![]() Na2CO3+CO2��+H2O�����Բ���˵����Ʒ�к��ᾧˮ��Ca2++
Na2CO3+CO2��+H2O�����Բ���˵����Ʒ�к��ᾧˮ��Ca2++![]() ====CaCO3���ǽ���Ϥ��֪ʶ�㣬����
====CaCO3���ǽ���Ϥ��֪ʶ�㣬����![]() �Ĵ��ڣ�ע�ⲻ���ó���ʯ��ˮ�����������飬ԭ������
�Ĵ��ڣ�ע�ⲻ���ó���ʯ��ˮ�����������飬ԭ������![]() �ĸ��š�(2)�ڶ�������ʱ����������2NaHCO3
�ĸ��š�(2)�ڶ�������ʱ����������2NaHCO3![]() Na2CO3+CO2��+H2O ���ԭ�����������֪��װ��ʯ�ҵ�U�ι�D������������CO2����Ϊ75.8 g-74.7=1.1 g��n(CO2)��0.025 mol��
Na2CO3+CO2��+H2O ���ԭ�����������֪��װ��ʯ�ҵ�U�ι�D������������CO2����Ϊ75.8 g-74.7=1.1 g��n(CO2)��0.025 mol��
2NaHCO3![]() Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
2 1 1 1
0.05 mol0.025 mol0.025 mol0.025 mol
m��NaHCO3��=0.05 mol��84 g��mol-1=4.2 g
���Ծ�����![]() ��
��
m��H2O��=0.025 mol��18 g��mol-1=0.45 g
m(Na2CO3)��7.3 g-4.2 g-0.45 g��2.65 g��
![]() ���ɴ˿�֪������
���ɴ˿�֪������
n(Na2CO3)��n(NaHCO3)��n(H2O)��0.025 mol��0.05 mol��0.025 mol��1��2��1��
���Ծ��廯ѧʽΪNa2CO3��2NaHCO3��H2O��


 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������һģ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ϻ��з������߿���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1������2.49 g��Ʒ����ͬ����ʵ��ʱ������CO2___________mL����״������
��2����ȡ3.32 g��Ȼ����Ʒ��300 ����ȷֽ�����ȫ��300 �� ʱ Na2CO3���ֽ⣩������CO2 112 mL����״������ˮ0.45 g����ͨ������ȷ������Ȼ��Ļ�ѧʽ��
��3��������������ʵ���Ũ��Ϊ______________________��
��4����������ij��ʵ���У��ռ���336 mL����״�������壬�ô�ʵ���г�ȡ��Ȼ����Ʒ������������______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com