
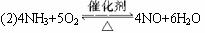
ʵ���������Լ����������з�Χ��
��NH4Cl��Ca(OH)2�Ļ���� ��4��1��ˮ ��NaOH���� ��KClO3��MnO2�Ļ���� ������ˮ��NH4HCO3���� ��6 mol��L-1 NaOH��Һ ��0.5 mol��L-1 NaOH��Һ ���̪��Һ ���ʯ�� ��ŨH2SO4 ?��CuO
������������⣺
��1���Ͳ��ι���Һ̬����A��__________����������B��__________��
��2��C��������Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��3�������D��Ϊ�˼����������ʵ����ɶ�����ģ��������__________��ʵ������е�������_______________________��
��4��E�����β����ܵ�������________________________________________��
��5��F������Ӧպȡ��������_________________________________���䷴Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��
��1��4��1�İ�ˮ����ڣ� KClO3��MnO2�Ļ�����ܣ�
![]()
(3)0.5 mol��L-1 NaOH��Һ�ͷ�̪��Һ�����͢ᣩ ��ɫ��ȥ
��4����ֹҺ̬����D���
��5��6 mol��L-1 NaOH��Һ����ߣ� NO2+NO+2NaOH====2NaNO2+H2O
������(1)��Ϊ��װ���ǰ��Ĵ����������鷴Ӧ���ɵ��������ʵ���װ�ã�����A��B��һ������O2�ģ�һ�����ư����ģ�������Ŀ��ʾBΪ��̬װ�ã������Լ���ֻ�Т�Ϊ��������ҩƷ��Ϊ��̬������B����KClO3��MnO2����AΪ�ư�����װ�á���ΪAΪҺ̬���ʣ�ֻ��ѡ�Լ��еĢڣ���4��1�İ�ˮ��ΪʹNH3�����ݳ���������NaOH���壩��
��2��������ά�����������NH3��O2�������ĽӴ������ʹ��Ӧ��ֵ����á�
��3��Ϊ�������ɵ��������壬����D�м����Һ�ͷ�̪��Һ��Ϊʹ��������ԣ�Ӧ����0.5 mol��L-1��NaOH��Һ������D�м���0.5 mol��L-1��NaOH��Һ�ͷ�̪��Һ�����Һ�ʺ�ɫ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�ͻ�ѧʵ�飬����װ��С�ɣ���ԼҩƷ��������㣬�������ԣ���ȫ�ɿ���������Ⱦ���ص㣬��Խ��Խ�ܵ����ǵ����ӡ���ͼ��ijͬѧ���NH3�Ĵ����������鷴Ӧ���ɵ��������ʵ���װ��(ͼ�б�Ҫ������̨�����С��;ƾ��ƵȾ�����ȥ)
�����������Լ��������з�Χ��
��NH4Cl��Ca(OH)2�Ļ�����4��1�İ�ˮ����Na2O2����KClO3��MnO2��������ˮ����NH4HCO3���壻��6mol/L NaOH��Һ����0.5mol/L NaOH��Һ������̪��Һ������ʯ�ң���ŨH2SO4����CuƬ���Իش��������⣺
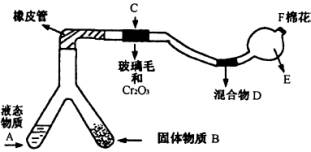
(1)�Ͳ��ι���Һ̬����A��____ ___����������B��_�������� ______��
(2)C��������Ӧ�Ļ�ѧ����ʽ��__�������������������������������� _____��
(3)�����D��Ϊ�˼����������ʵ����ɶ������ģ��������_���������������� ___���������������� ___��ʵ������е�������____�������������������� ___��
(4)E�����β����ܵ�������_____������������������������ __��
(5)F������Ӧպȡ��������_____���� __����Ӧ�Ļ�ѧ����ʽ��___������������ ___�������������������������������� _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����ʽ����½̰��������л�ѧ�����꼶���ڶ��ᣨ�ϲᣩ ���ͣ�058
�ͻ�ѧʵ�����װ��С�ɣ���ԼҩƷ��������㣬�������ԣ���ȫ�ɿ���������Ⱦ���ص㣮��ͼ��ijͬѧ��Ƶ�NH3�����������鷴Ӧ���ɵ��������ʵ���װ��(ͼ�б�Ҫ������̨�����С��;ƾ��ƵȾ�����ȥ)��
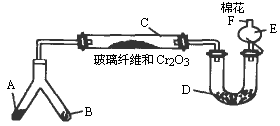
ʵ���������Լ����������з�Χ��
��NH4Cl��Ca(OH)2�Ļ�����4��1��ˮ����NaOH���壻��KClO3��MnO2�Ļ���������ˮ����NH4HCO3���壻��6 mol/L NaOH��Һ������0.5 mol/L NaOH��Һ�����̪��Һ�����ʯ�ң�![]() ŨH2SO4��
ŨH2SO4��![]() CuO���Իش��������⣺
CuO���Իش��������⣺
(1)�Ͳ��ι���Һ̬����A��________����B��________��
(2)C��������Ӧ�Ļ�ѧ����ʽ��________��
(3)�����D��Ϊ�˼����������ʵ����ɶ�����ģ��������________��ʵ������е�������________��
(4)E�����β����ܵ�������________��
(5)F�Դ�����Ӧպȡ��������________���䷴Ӧ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����ṩ��ҩƷ�У���NH4Cl��Ca��OH��2�Ļ����ڰ�ˮ����KClO3��MnO2��������ˮ����0.5 mol/L NaOH��Һ����̪��Һ����ŨH2SO4����6 mol/L NaOH��Һ��

������������⣺
��1���Ͳ��ι���Һ̬����A��_____________����������B��_____________��
��2��C��������Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��3�������D��Ϊ�˼����������ʵ����ɶ�����ģ��������_______________��ʵ������е�������_____________��
��4��F������Ӧպȡ��������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ͻ�ѧʵ�����װ��С�ɣ���ԼҩƷ��������㣬�������ԣ���ȫ�ɿ���������Ⱦ���ص㣬��ͼ��ijͬѧ��Ƶ�NH3�Ĵ����������鷴Ӧ���ɵ��������ʵ���װ�ã�ͼ�б�Ҫ������̨�����С��;ƾ��ƵȾ���ȥ����
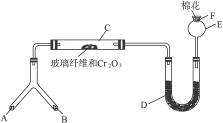
ʵ���������Լ����������з�Χ��
��NH4Cl��Ca(OH)2�Ļ���� ��4��1��ˮ ��NaOH���� ��KClO3��MnO2�Ļ���� ������ˮ��NH4HCO3���� ��6 mol��L-1 NaOH��Һ ��0.5 mol��L-1 NaOH��Һ ���̪��Һ ���ʯ�� 11ŨH2SO4 ?12CuO
������������⣺
��1���Ͳ��ι���Һ̬����A��__________����������B��__________��
��2��C��������Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��3�������D��Ϊ�˼����������ʵ����ɶ�����ģ��������__________��ʵ������е�������_______________________��
��4��E�����β����ܵ�������________________________________________��
��5��F������Ӧպȡ��������_________________________________���䷴Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com