
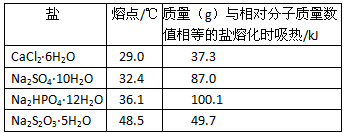
�� | �۵�/�� | ������g������Է���������ֵ��ȵ����ۻ�ʱ����/kJ |
CaCl2��6H2O | 29.0 | 37.3 |
Na2SO4��10H2O | 32.4 | 87.0 |
Na2HPO4��12H2O | 36.1 | 100.1 |
Na2S2O3��5H2O | 48.5 | 49.7 |
�����й�˵����ȷ���ǣ� ��
�ٲ���ѡ��CaCl2��6H2O �ڿ�ѡ��Na2SO4��10H2O��Na2HPO4��12H2O �����ѡ��Na2S2O3��5H2O �����ѡ��Na2SO4��10H2O
A.�٢� B.�ڢ� C.�٢� D.�ڢ�
����������������Դ����Ϊ��������㣨�¿�������֪ʶ�㣩���ۺϿ����ж������������Ա������ͼ�����������ϸ�Ľ�����Ϊ��ѡ����ִ��ܽ���һ��Ҫ���������������棺һ�Ǿ����ԣ�����ԴҪ�ḻ������ȡ�ã��۸���ˣ�����ͬ�������Ĵ��ܽ��ʵĴ���ֵ���ۻ�ʱ���������������á����и����ʵ�λ������g���ۻ�ʱ���յ���������Ϊ��
CaCl2��6H2O(��Է�������219):![]() =0.17 kJ��g-1
=0.17 kJ��g-1
Na2SO4��10H2O(��Է�������322):![]() =0.27 kJ��g-1
=0.27 kJ��g-1
Na2HPO4��12H2O(��Է�������358):![]() =0.28 kJ��g-1
=0.28 kJ��g-1
Na2S2O3��5H2O����Է�������248����![]() =0.20 kJ��g-1
=0.20 kJ��g-1
����Ȼ��Na2SO4��10H2O��Na2HPO4��12H2O���ߵ�λ�����ۻ�ʱ���������ϴ������µ�Ч���Ϻã������ѡ��Na2SO4��10H2O(â��)����������Ȼ���зֲ��㷺����Ҫ�ֲ����κ��ͺ�ˮ������ҹ���ʢ����أ��۸���ˣ�ʹ���������á�
�𰸣�D


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��0115 ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com