
��֪H2(g)+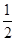 O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������
O2(g)=H2O(g)����H=һ24l.8kJ/mol����˵���д������
| A��H2��ȼ����Ϊ241.8kJ/mol |
| B��2H2(g)+O2(g)=2H2O(g)����H=-483.6kJ/mol |
| C��1mol H2��ȫȼ������Һ̬ˮ�ų�����������24l.8kJ |
| D���Ͽ�1molH2O�Ļ�ѧ�����յ����������ڶ���lmolH2��0.5molO2�Ļ�ѧ�������յ������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)��1����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/( kJ��mol��1) | ��283.0 | ��285.8 | ��726.5 |
��ش��������⣺�ٸ�������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��__________________________��
�ڸ��ݸ�˹����������з�Ӧ���Ȼ�ѧ����ʽ��CO(g)+2H2(g)===CH3OH(l)��H=____________��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
�� CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H1=+49.0 kJ��mol-1
�� CH3OH(g)+O2(g)=CO2(g)+2H2(g) ��H2
��֪H2(g)+ O2(g)===H2O(g) ��H = -241.8 kJ��mol-1
��Ӧ�ڵġ�H2 = kJ��mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���¡��״����� ��������������������������ĵ缫��ӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)��1����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/( kJ��mol��1) | ��283.0 | ��285.8 | ��726.5 |
��ش��������⣺�ٸ�������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��__________________________��
�ڸ��ݸ�˹����������з�Ӧ���Ȼ�ѧ����ʽ��CO(g)+2H2(g)===CH3OH(l)��H=____________��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
�� CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H1=+49.0 kJ��mol-1
�� CH3OH(g)+O2(g)=CO2(g)+2H2(g) ��H2
��֪H2(g)+ O2(g)===H2O(g) ��H = -241.8 kJ��mol-1
��Ӧ�ڵġ�H2 = kJ��mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���¡��״����� ��������������������������ĵ缫��ӦΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��������ѧУ�߶���ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
(9��)��1����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/( kJ��mol��1) | ��283.0 | ��285.8 | ��726.5 |
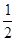 O2(g)= CO2(g)+2H2(g) ��H2
O2(g)= CO2(g)+2H2(g) ��H2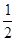 O2(g)===H2O(g) ��H =" -241.8" kJ��mol-1
O2(g)===H2O(g) ��H =" -241.8" kJ��mol-1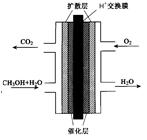
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��13�֣�ֱ�Ӽ״�ȼ�ϵ�أ�DNFC������Ϊ��21���͵綯������Ѻ�ѡ����Դ��
��1��101 kPaʱ��1 mol CH3OH��ȫȼ�������ȶ���������ų�����726.51 kJ/mol����״�ȼ�յ��Ȼ�ѧ����ʽΪ ��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ����H1=+49.0 kJ��mol-1
��CH3OH(g)+ O2(g)= CO2(g)+2H2(g)����H2
O2(g)= CO2(g)+2H2(g)����H2
��֪H2(g)+ O2(g)===H2O(g)������H =-241.8 kJ��mol-1
O2(g)===H2O(g)������H =-241.8 kJ��mol-1
��Ӧ�ڵġ�H2= ������������������ ��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���ҡ��״�������������������������������ü������ĵ缫��ӦΪ�������� ������������ ������

��4����֪H��H����Ϊ436 KJ/mol��H��N����Ϊ391KJ/mol�����ݻ�ѧ����ʽ��
N2 ��g��+ 3H2 ��g��= 2NH3��g�� ��H= ��92.4 KJ/mol����N��N���ļ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��������̨���и߶���ѧ����ĩ��ǰģ�⻯ѧ�Ծ� ���ͣ������
(9��)��1����֪25�桢101kPaʱ��һЩ���ʵ�ȼ����Ϊ��
|
��ѧʽ |
CO(g) |
H2(g) |
CH3OH(l) |
|
��H/( kJ��mol��1) |
��283.0 |
��285.8 |
��726.5 |
��ش��������⣺�ٸ�������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��__________________________��
�ڸ��ݸ�˹����������з�Ӧ���Ȼ�ѧ����ʽ��CO(g)+2H2(g)===CH3OH(l)��H=____________��
��2���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ�
�� CH3OH(g)+H2O(g)=CO2(g)+3H2(g) ��H1=+49.0 kJ��mol-1
�� CH3OH(g)+ O2(g)=
CO2(g)+2H2(g) ��H2
O2(g)=
CO2(g)+2H2(g) ��H2
��֪H2(g)+  O2(g)===H2O(g)
��H = -241.8 kJ��mol-1
O2(g)===H2O(g)
��H = -241.8 kJ��mol-1
��Ӧ�ڵġ�H2 = kJ��mol-1��
��3���״�ȼ�ϵ�صĽṹʾ��ͼ���¡��״����� ��������������������������ĵ缫��ӦΪ ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com