
����Ŀ��(1)��֪2 mol����ȼ������Һ̬ˮʱ�ų�572 kJ����������Ӧ����ʽ��2H2(g)��O2(g)===2H2O(l)����ش��������⣺
(1)�ٸ÷�Ӧ�������������ܺ�________(����ڡ�����С�ڡ����ڡ�)��Ӧ�������ܺ͡�
����2 mol������ȫȼ������ˮ��������ų�������________(�>������<������)572 kJ��
(2)FeS2���ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)��O2(g) ![]() 2SO3(g)����H1����197 kJ��mol��1
2SO3(g)����H1����197 kJ��mol��1
H2O(g)===H2O(l)����H2����44 kJ��mol��1
2SO2(g)��O2(g)��2H2O(g)===2H2SO4(l)����H3����545 kJ��mol��1
��SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��_________________ ��
(3)��֪���з�Ӧ���Ȼ�ѧ����ʽ��
��6C(s)��5H2(g)��3N2(g)��9O2(g)===2C3H5(ONO2)3(l)����H1
��2H2(g)��O2(g)===2H2O(g)����H2
��C(s)��O2(g)===CO2(g)����H3
��Ӧ4C3H5(ONO2)3(l)===12CO2(g)��10H2O(g)��O2(g)��6N2(g)�Ħ�HΪ________��
���𰸡� С�� < SO3(g)��H2O(l)===H2SO4(l) ��H����130 kJ��mol��1 12��H3��5��H2��2��H1
��������(1)������ȼ���Ƿ��ȷ�Ӧ����˸÷�Ӧ�������������ܺ�С�ڷ�Ӧ�������ܺ͡���ˮ������������Һ̬ˮ�����������2 mol������ȫȼ������ˮ�����ų���������572 kJ��
(2)��֪��
��2SO2(g)��O2(g)![]() 2SO3(g)����H1����197 kJ��mol��1
2SO3(g)����H1����197 kJ��mol��1
��H2O(g)��H2O(l)����H2����44 kJ��mol��1
��2SO2(g)��O2(g)��2H2O(g)��2H2SO4(l)����H3����545 kJ��mol��1
����ݸ�˹���ɿ�֪���ۣ��٣��ڡ�2��/2���õ�SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��SO3(g)��H2O(l)��H2SO4(l)����H����130 kJ��mol��1��
(3)��֪��
��6C(s)��5H2(g)��3N2(g)��9O2(g)��2C3H5(ONO2)3(l)����H1
��2H2(g)��O2(g)��2H2O(g)����H2
��C(s)��O2(g)��CO2(g)����H3
����ݸ�˹���ɿ�֪�ۡ�12+�ڡ�5���١�2���õ���Ӧ4C3H5(ONO2)3(l)��12CO2(g)��10H2O(g)��O2(g)��6N2(g)�Ħ�H��12��H3��5��H2��2��H1��


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A. ʵ�����������ữ���������Һ
B. ���ƻ�������������ʢװ�ȵ�Ũ����
C. �����£��������ȷ�Ӧ�������Է�����
D. ��ȥMgCl2��Һ������FeCl3���ʣ��ɼ���������MgO��ĩ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡�
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��
C(s)��H2O(g)![]() CO(g)��H2(g) ��H����131.3 kJ��mol��1
CO(g)��H2(g) ��H����131.3 kJ��mol��1
��ʹ��ѧ��Ӧ���ʼӿ�Ĵ�ʩ��________(�����)��
������C�����ʵ��� �����߷�Ӧ�¶�
����ʱ����CO��H2ת��ΪCH3OH ���ܱն��������г���CO(g)
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)CO2(g)��H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
2 | 900 | 2 | 4 | 0.8 | 3.2 | 3 |
��ʵ��1����v(CO2)��ʾ�Ļ�ѧ��Ӧ����Ϊ________��
�ڸ÷�Ӧ���淴ӦΪ________(������š�)�ȷ�Ӧ��
(3)��һ�ݻ�Ϊ2 L���ܱ������ڼ���2 mol��CO��6 mol��H2����һ�������·������·�Ӧ��CO(g)��2H2(g) ![]() CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
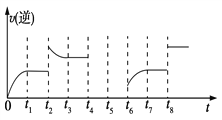
����ͼ��֪��Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬����t2��t8ʱ���ı������������ж�t8ʱ�ı������������________��
����t4ʱ��ѹ��t5ʱ�ﵽƽ�⣬t6ʱ����Ӧ���Ũ�ȣ�����ͼ�л���t4��t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ���ߡ�________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϴӺ�ˮ����ȡ�����Ҫ��Ӧ�ǣ�C12+2Br- = 2Cl- + Br2����˵����������
A����ˮ����Ԫ����Ҫ����ʽBr-���� B��������Ӧ�������ӷ�Ӧ
C���嵥�ʱ��ȵ��ʻ��� D��������Ӧ����������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л�����ⷴӦ������(CH3)2CHCH2OH�����л��������
A.CH2=C(CH3)CH2OHB.CH3CH2CH2CHO
C.(CH3)2CHCOOHD.(CH3)3CCHO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ��һ�ֺ�ɫ���壬������ϡ���ᡣijͬѧ��֪��ϡ�����е���������(H2O��H����SO![]() )��ʹ����ͭ�ܽ⡣�������һ��ͨ����ͼ��ʾ������͢�����ʵ��������̽�����
)��ʹ����ͭ�ܽ⡣�������һ��ͨ����ͼ��ʾ������͢�����ʵ��������̽�����

(1)ͨ��ʵ������֤��____________________________________��
(2)Ҫ֤���������������ܷ��ܽ�����ͭ������Ҫ����ʵ���͢��ڢ��м���ϡ���������ͭ�ܽ⣬���һ��ȷ�ϵ�ʵ������ǣ��ڢ����ȼ���________���ټ���________��
(3)̽�����Ϊ_____________________________________________��
(4)��Ӧ�����ӷ���ʽΪ____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ǽ����л����ת����ϵ���밴Ҫ����գ�

��֪��A������ʯ�͵���Ҫ�л�����ԭ�ϣ���ˮ���д������ã�75%��B������ҽ��������E�� ���й���ζ���л���ṹ��ʽΪC4H8O2��F��һ�ָ߾�����Ƴɶ��ְ�װ���ϡ�
��1��G��������_____________�� F��������_____
��2����Ӧ���ͣ���__________�� ��_____
��3����д��Ӧ����ʽ�� ��_____
��4�����й��� A �� F ��������ȷ����_____
A��A �����������壬Ϊ�����F �������ǹ��壬Ϊ�����
B��A �� F ����ʹ���Ը��������Һ��ɫ
C��ȡ�������� A �� F ��ȫȼ�պ����ɵ� CO2 �� H2O �������ֱ����
D��ȡ�����ʵ����� A �� F ��ȫȼ�պ����ɵ� CO2 �� H2O �����ʵ����ֱ����
��5���� E ��Ϊͬ���칹�壬�Һ�������COO�����ṹ���л��ﻹ��____________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ����ʽCl2 + 2I- = 2Cl- + I2��˵������ȷ����
A.Cl2��������B.I2�ǻ�ԭ����
C.������Ӧ�����û���ӦD.������Ӧ�������ӷ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com