
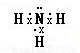 ���ʴ�Ϊ�����Է��ӣ�
���ʴ�Ϊ�����Է��ӣ�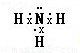 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��
��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������2011��2012ѧ���һ��ѧ��12���¿���ѧ���� ���ͣ�022
��ǰ18��Ԫ����ɵĵ���A��B��C(����AΪ����)�ͼס��ҡ����������ֻ���������ͼ��ת����ϵ����֪CΪ�ܶ���С�����壬���ǵ���ʣ�
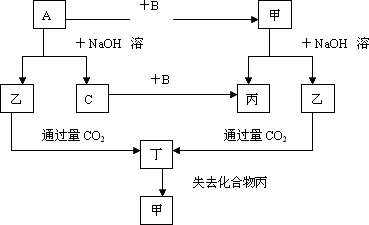
��������ת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��
A��________B��________�ң�________����________
(2)��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��________��
�����������CO2��Ӧ�����ӷ���ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����������һ��2010������߿�ģ�������⣨�����ۺϣ���ѧ���� ���ͣ������
��10�֣� A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮��������·�Ӧ��ϵ��

��1������ת�������а����ķ�Ӧ����Ϊ ��
a�û���Ӧ b���Ϸ�Ӧ c�ֽⷴӦ d���ֽⷴӦ
��2�����ڼ���ȼ�գ�����1 mol e��ת�ƣ�����Һ̬Aʱ�� �ų�142.9 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����Ӧ����һ��������Ϊ���淴Ӧ������һ��ʱ��÷�Ӧ�ﵽƽ�⣬д���÷�Ӧ�Ļ�ѧ����ʽ�� ������3 mol��4 mol C��ϳ����ݻ�Ϊ2 L���ܱ������У�2���Ӻﵽƽ�⡣ƽ������������ʵ���Ϊ6 mol���Ļ�ѧ��Ӧ����Ϊ ������ƽ�����ﻺ��ͨ��ˮ�еõ�1L��Һ��������Һ���ʵ���Ũ����____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com